Abstract
The corrosion inhibition study of 2-chloro 3-formyl quinoline was conducted for mild steel corrosion in 1 M hydrochloric acid solution at a temperature range of 303–333 K by chemical and electrochemical measurements. Inhibition efficiency increases with the increase of inhibitor concentration but decreases with increasing temperature. The polarization measurement reveals that the inhibitor acts as the mixed type and this inhibition effect is attributed to the adsorption of the inhibitor on the surface of mild steel from the bulk of the solution. The adsorption of 2-chloro 3-formyl quinoline on mild steel surface is exothermic and obeys the Freundlich adsorption isotherm. Thermodynamic parameters and activation parameters were calculated and discussed in depth. Scanning electron microscopy (SEM) micrographs were used to investigate the surface morphology of the steel sample in presence and absence of inhibitor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mild steel is one of the most important alloys of iron, which has the wide range of structural and industrial applications. The corrosion of iron and steel is of fundamental, academic and industrial concern that has received a considerable amount of attention [1]. The mild steel is handled under acids, alkalis and salt solutions in various industrial processes. Therefore, under these conditions chlorides, sulfates and nitrates are aggressive and cause corrosion. Various corrosion controlling methods were used to protect the metals such as protective coatings, cathodic protection and the use of corrosion inhibitors. Among these methods, the use of corrosion inhibitors is the most convenient and practical method to protect the metals from attack of corrosion [2]. In this method, corrosion inhibitor molecules get adsorbed on the surface of the mild steel from the bulk of the solution, which blocks the active corrosion sites, which retards the corrosion [3].
2-Chloro 3-formyl quinoline was selected as a corrosion inhibitor for mild steel corrosion in 1 M HCl, which is a heterocyclic organic compound that consists of electron-rich species such as nitrogen, oxygen and π electrons in the heterocyclic ring system. Inhibitor molecule is planar in its structure and free from toxic groups, which gives scope to study it as a potential corrosion inhibitor for mild steel in 1 M HCl [4]. Corrosion inhibition effect of the inhibitor on corrosion of mild steel in 1 M HCl has been investigated by weight loss, electrochemical Tafel polarization and electrochemical impedance spectroscopy (EIS) measurements.
Experimental
Materials
Mild steel strips (composition: 0.16 % C, 0.35 % Mn, 0.016 % Si, 0.01 % P, 0.029 % S, 0.06 % Cr, 0.1 % Cu and the remaining is Fe) with a dimension of 5 cm × 1 cm × 0.1 cm were used for weight loss method, and the same strips with an exposed area of 1 cm2 (remaining portion was insulated by resin) were used for electrochemical measurements. The strips were abraded with emery papers from grade number 80 up to 2000. AR grade hydrochloric acid and double-distilled water were used to prepare the 1-M HCl corrosive media for all the experiments.
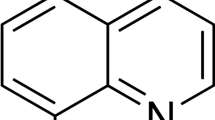
2-Chloro 3-formyl quinoline is having molecular weight 191.61 and a melting point of 144 °C, which is soluble in ethanol. For all the experiments, the inhibitor was first dissolved in 2 ml of ethanol and then added to HCl media. The molecular structure of 2-chloro 3-formyl quinoline is as shown in Fig. 1.
Methods
Weight loss measurement
Mild steel strips of different weights were immersed in different beakers containing 100 ml of 1 M hydrochloric acid solutions(with 2 ml ethanol) in the absence and presence of different concentrations such as 50, 100, 150 and 200 ppm of 2-chloro 3-formyl quinoline for about 4 h immersion time at room temperature. Mild steel strips were weighed after and before immersion time to record the weight difference. By the help of weight differences corrosion rate and inhibition, efficiency was calculated.
Tafel polarization measurement
Tafel polarization measurement was carried out with the use of three electrode system such as the working electrode (mild steel strip), a counter electrode (platinum) and a reference electrode (SCE) by using a CHI608D electrochemical workstation at a temperature range of 303-333 K. In this measurement potential–current curves were recorded at a scan rate of 0.001 V/s in the given potential range.
Electrochemical impedance spectroscopic (EIS) measurement
The electrochemical impedance spectroscopy (EIS) measurement was carried out for the mild steel corrosion in 1 M HCl at a temperature range of 303–333 K. In this measurement, impedance spectra were recorded by AC signals with amplitude of 5 mV/s at OCP in the frequency range from 0.1 kHz up to 1 Hz.
Thermodynamic parameters
Thermodynamic parameters were computed by evaluating the proper adsorption isotherm model fit for the adsorption of the inhibitor on the surface of the mild steel. In this present work data obtained by EIS method was used to evaluate the adsorption isotherm.
Activation parameters
Activation parameters of the inhibitor were computed and discussed for the mild steel corrosion by using Arrhenius and transition theory. The data obtained from electrochemical Tafel polarization method were used to evaluate the activation parameters at the temperature range of 303–333 K.
Scanning electron microscopy (SEM)
The SEM micrographs of mild steel in the absence and presence 2-chloro 3-formyl quinoline for about 4 h immersion period in 1 M HCl were recorded using scanning electron microscopy (JEOL JSM-840A model).
Result and discussion
Weight loss measurement
The values of corrosion rate (CR) and the inhibition efficiency (\(\eta_{w}\)) of inhibitor for mild steel corrosion in 1 M HCl were obtained from weight loss measurement in the absence and presence of various inhibitor concentrations as reported in Table 1. The inhibition efficiency (\(\eta_{w}\)) was calculated by the following expression:
where ν and ν i are the corrosion rates of mild steel in the presence and absence of inhibitors in the solution, respectively.
Corrosion rate (CR) of the mild steel increases with the increasing of inhibitor concentration (Table 1) due to the blocking of active corrosion sites by the inhibitor molecules on the surface of the mild steel. The inhibition efficiency (\(\eta_{w}\)) of inhibitor increases with the increasing inhibitor concentrations of 50–200 ppm. The inhibition efficiency of QMC (2-chloro quinoline 3-carbaldehyde) for mild steel in 1 M HCl was reported as 80 % [5]. Therefore, the present work shows that the 2-chloro 3-formyl quinoline acts as an efficient inhibitor with maximum inhibition efficiency of 88.22 %. This behavior can occur due to the strong interaction of the inhibitor with the metal surface by adsorption. Therefore, continuous adsorption of inhibitors on the surface of mild steel covers the surface area to protect the metal from attack by corrosion [6].
Tafel polarization measurement
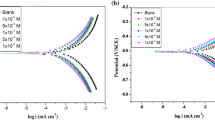
The Tafel polarization plots were recorded for the mild steel corrosion in the absence and presence of 2-chloro 3-formyl quinoline in 1 M HCl solution at a temperature range of 303–333 K as shown in Fig. 2.
The computed corrosion parameters such as corrosion potential (E corr), corrosion current density (i corr), corrosion rate (CR) and inhibition efficiency (η p ) are reported in Table 2. The inhibition efficiency (η p ) was calculated using the following equation:
where \(i_{\text{corr}}^{0}\) and i corr are the corrosion current density in the absence and presence of inhibitor, respectively.
The results obtained by Tafel polarization method reveals that the corrosion current density (icorr) decreases with the addition of inhibitor. But the value of increases with increasing temperature because at elevated temperature desorption takes place over the metal surface. It implies that this system reduces the inhibition efficiency of the inhibitor for the corrosion of mild steel in 1 M HCl solution at elevated temperatures. The corrosion inhibitor is usually classified as anodic or cathodic type when the change in corrosion potential (E corr) value is greater than 85 mV. The largest displacement in E corr value on the blank was 40 mV (Table 2) after the addition of 2-chloro 3-formyl quinoline, indicating that the inhibitor acts as a mixed type inhibitor [7, 8].
From the Table 2, corrosion rate (CR) of the inhibited solution gradually decreases with respect to the uninhibited solution. Therefore, the adsorption of the inhibitor molecule get blocks the corrosion active sites of the mild steel surface, which retards the corrosion.
It is found that the inhibition efficiency of the some important quinoline derivatives such as CQMFA was 84 % at room temperature [9]. But in the present study, 2-chloro 3-formyl quinoline shows better inhibition efficiency even at an elevated temperature up to 323 K. The maximum inhibition efficiency obtained is around 85 %.
Electrochemical impedance spectroscopic (EIS) measurement
The kinetics of the electrode processes and surface properties for the corrosion inhibition study of mild steel by 2-chloro 3-formyl quinoline in 1 M HCl solution was investigated by the electrochemical impedance spectroscopic measurement. The Nyquist plots and Bode plots were recorded for mild steel in the absence and presence of 2-chloro 3-formyl quinoline in 1 M HCl at a temperature range of 303–333 K as shown in Figs. 3 and 4, respectively. An equivalent circuit model as shown in Fig. 5 was used to fit and analyze EIS data. The experimental curve was exactly fitted with the curve obtained by the electrical equivalent circuit as shown in Fig. 6. This circuit consists of polarization resistance (R p), which is proportional to the diameter of the semicircle, solution resistance (R s) and capacitance of the double layer (C dl). The computed corrosion parameters from this measurement such as polarization resistance (R p), double-layer capacitance (C dl) and the calculated inhibition efficiency (η z ) are reported in Table 2.
The Nyquist plots consist of a depressed semicircle in the complex impedance plane with the center of the real axis and the depressed loops increased with the increasing inhibitor concentration. R p value decreases with the increasing temperature due to the desorption process from the mild steel surface to the bulk of the solution. The obtained semicircles are not perfect semicircles; this is because of the typical behavior of a solid metal electrode that shows frequency dispersion of the impedance data [10, 11] which is attributed to the roughness and other inhomogeneities of the solid surface [12–14].
The inhibition efficiency (η z ) increases with increasing of inhibitor concentration with the range of 50–200 ppm. This increasing inhibition efficiency is attributed to the formation of a protective layer on the surface of the mild steel. As a result, the 200 ppm of 2-chloro 3-formyl quinoline exhibits maximum inhibition efficiency of around 85 % up to 323 K temperature. Inhibition efficency decreases at 333 K. This is because the desorption of inhibitor takes place from the surface of the mild steel. The obtained inhibition efficiency of CQC and CQA quinoline derivatives was found as to be 93.88 and 99.06 %, respectively, by this EIS method. In the present study, the inhibitor shows excellent inhibition efficiency even at elevated temperatures up to 313 K.
From the Bode plots in Fig. 4, the depressed semicircles usually obtained for an electrode/solution interface due to the rough electrode surface. The corrosion of mild steel in acid media increases the roughness of the electrode surface and, therefore, reduces the phase angle [15]. In Fig 4, the phase angle increases with increase in inhibitor concentration up to 313 K and it decreased up to 333 K temperature due to desorption of inhibitor molecule.
Inhibition efficiency (η z ) was calculated using the following equation [16]:
where R p and \(R_{\text{p}}^{0}\) are the polarization resistance values in the presence and absence of inhibitor. The double-layer capacitance values (C dl) were evaluated by the following formula:
where Q is the constant phase element (CPE) (Ω−1 Sn cm−2) and n is the CPE exponent.
Figure 2 shows that the electrochemical impedance spectrum (EIS) consists of semicircles with their centers on the real axis and that the diameter of the semicircles increases with the increase in the inhibitor concentration in the range of 50–200 ppm. This is the indication of the adsorption of the inhibitor molecule on the metal surface [17]. The difference in real impedance at lower and higher frequencies is commonly considered as a charge-transfer resistance [18]. But the R p value decreases with the increasing of the temperature, indicating that the desorption of the inhibitor molecule from the metal surface plays a major role rather than that of the adsorption process. According to the Table 2 the C dl values are decreased with increase in inhibitor concentration due to either the decrease in dielectric constant or increase in the thickness of the electric double layer, suggesting that the 2-chloro 3-formyl quinoline is adsorbed at the metal/solution interface.
Inhibition efficiency obtained by the EIS measurement showed good agreement with the result obtained from Tafel and weight loss measurements. While considering both the measurements, it could be observed that the 2-chloro 3-formyl quinoline acts as a good corrosion inhibitor and shows better inhibition efficiency around 85 % or mild steel in 1 M HCl.
Thermodynamic parameters
Basically, inhibition effect of the inhibitors in acid solutions occurs due to the adsorption on the surface of metal [19]. The adsorbed inhibitor molecules retard both the anodic and cathodic electrochemical corrosion reactions, which reduces the corrosion rate. This interaction in between the inhibitor and metal surface can be studied by adsorption isotherm model. The adsorption process obeys the Freundlich adsorption isotherm. According to this isotherm surface coverage can be calculated by the following equation
where η z is the inhibition efficiency obtained from electrochemical impedance spectroscopy (EIS). The adsorption of inhibitor was attributed to the interaction in between the surface coverage (θ) and inhibitor concentration (C) in millimoles. This relationship is described as follows:
where K ads is the equilibrium constant of the adsorption process. K ads value is directly related to the standard free energy of adsorption (\(_{\text{ads}}^{0}\)). Plot a graph of C/θ against the inhibitor concentration (C), which is shown in Fig. 7. The obtained plot consists of a straight line with the regression coefficient (R 2) which is almost near to unity.
K ads values can be calculated by the slope (K ads = 1/C) of the straight lines on the C/θ axis. K ads values represent a strong interaction between the adsorbed inhibitor molecule and the metal surface. Kads value indicates that, better adsorption with good inhibition efficiency at higher temperature [20]. The relationship between K ads and \(\Delta G_{\text{ads}}^{0}\) is expressed as follows:
where the R is the universal gas constant value of 8.314 kJ/kg/K, T is absolute temperature, and 55.5 is the molar concentration of water in the bulk of the solution. The calculated values of K ads and \(\Delta G_{\text{ads}}^{0}\) are reported in Table 3. The negative values of \(\Delta G_{\text{ads}}^{0}\) indicate the spontaneous adsorption process and stability of the adsorbed layer on the mild steel surface.
The calculated ΔG ads values are within −40 and −20 kJ/mol, indicating that the adsorption mechanism of 2-chloro-3-formyl quinoline on surfaces of the mild steel in 1 M HCl solution at 303–333 K temperatures was a combination of both physisorption and chemisorption [21, 22]. The increasing value of K ads and \(\Delta G_{\text{ads}}^{0}\) with the increasing temperature as on 303–333 K indicates the consistent and stable adsorption of 2-chloro 3-formyl quinoline on mild steel surface in 1 M HCl solution.
Graph of ΔG ads/T vs 1/T were given in Fig. 8. This plot consists of a straight line with a regression coefficient as 0.998. The slope is equal to the standard enthalpy of adsorption (\(\Delta H_{\text{ads}}^{0}\)). The negative sign of \(\Delta H_{\text{ads}}^{0}\) in HCl solution indicates that the adsorption of inhibitor molecule is an exothermic process [23].
The positive value of entropy (\(\Delta S_{\text{ads}}^{0}\)) indicates that the reaction suffers a loss of a degree of freedom during the complex process. Also, as the adsorption process was exothermic, it should have been accompanied by a decrease in entropy. We found that the value of \(\Delta S_{\text{ads}}^{0}\) decreased with the increase of temperature as at 303–333 K.
Activation parameters
The activation parameters are the useful tool to understand the inhibition mechanism for mild steel by inhibitor molecule in acid media at the temperature range of 303–333 K. The electrochemical Tafel polarization data were used for the study of activation parameters at a temperature of 303–333 K for the mild steel in the absence and presence of 2-chloro 3-formyl quinoline in 1 M HCl. The energy of activation (E a) for the mild steel can be described using Arrhenius equation:
where E a is the energy of activation (J/mol), R is the gas constant (8.314 J/mol/K), T is the absolute temperature (K), A is the Arrhenius pre-exponential factor and υ corr is the corrosion rate. Arrhenius graph of ln υ corr vs 1/T as shown in Fig. 9. Plots are consist of linear with regression coefficient almost near to unity. The activation energy (E a) were calculated and reported in Table 4.
As per Arrhenius equation, the corrosion rate (υ corr) is being affected by the activation energy (E a). From Table 4, it is observed that E a value increases with the increasing inhibitor concentration. Which indicated that, Inhibitor molecule strongly adsorbed on the metal and it controls charge transfer on the metal surface. It suggests that metal dissolution is controlled by surface reaction [24]. The values of the standard enthalpy (ΔH*) and standard entropy (ΔS*) of activation were calculated using the following equation:
where h is plank’s constant (6.626 × 10−34) and Avogadro’s number (6.022 × 1023)
A transition plot of ln (υ corr/T) vs 1/T gave straight lines is depicted in Fig. 10, the ΔH* value calculated by using slope and ΔS* calculated by the help of intercept. The calculated values of ΔH* and ΔS* are listed in Table 4.
The increase in the enthalpy of activation [ΔH*] indicates that, addition of inhibitor increase the energy barrier for the corrosion reaction without changing dissolution mechanism. Negative value of entropy of activation [ΔS*] represents rate determining step with association rather than the dissociation step [25]. In addition to that the less negative values of ΔS* in the presence of inhibitor imply that the presence of inhibitor created a near-equilibrium corrosion system.
Scanning electron microscopy (SEM)
Scanning electron microscopic (SEM) images were taken to investigate the surface study the on the mild steel surface for the corrosion in the presence and absence of 2-chloro 3-formyl quinoline for an immersion period of 4 h at 303 K are shown in Fig. 11.
SEM image of the mild steel sample has rough surface (11 A) and it is completely covered by the inhibitor which forms smooth surface (11 B) [26].
Mechanism of inhibition
The 2-chloro 3-formyl quinoline drug molecules contain nitrogen, oxygen and fused benzene rings. The present work indicates that this inhibitor is adsorbed on the metal surface predominantly by chemisorption method. 2-chloro 3-formyl quinoline gets adsorbed on the mild steel surface by donor–acceptor interactions with the vacant d-orbital of metal. Nitrogen and oxygen atoms of the inhibitor may donate a lone pair of electrons to the vacant d orbital of the metal and forms co-ordinate bond. Also, π electrons of the aromatic rings also may form the same type of bond with the metal atom.
Conclusions
2-Chloro 3-formyl quinoline acts as a good corrosion inhibitor with the maximum inhibition in and around 80 %, corresponding to the optimum concentration at 200 ppm. The inhibition effect of 2-chloro 3-formyl quinoline is attributed to the adsorption process. This inhibitor acts as a mixed type inhibitor. Therefore, adsorption process is exothermic in nature which obeys the Freundlich adsorption isotherm. SEM micrograph gives a visual idea about the formation of a protective layer on the mild steel surface, which retards the corrosion rate. So 2-chloro 3-formyl quinoline shows to be a good inhibitor, as proved by all the chemical and electrochemical measurements.
References
Uhlig HH, Revie RW (1985) Corrosion and corrosion control. Wiley, New York
Liu J, Yu W, Zhang J, Hu S, You L, Qjao G (2010) Molecular modeling study on inhibition performance of imidazolines for mild steel in CO2 corrosion. Appl Surf Sci 256:4729–4733
Gomez B, Likhhanova NV, Dominguez-Aguilar MA, Martinez-Palou R, Vela A, Gazquez JL (2006) Quantum chemical study of the inhibitive properties of 2-pyridyl-azoles. J Phys Chem B 110:8928–8934
Matad Prasanna B, Mokshanatha Praveen B, Hebbar Narayana, Venkatesha Venkatarangaiah T, Tandon Harmesh Chander (2014) Ketosulfone drug as a green corrosion inhibitor for mild steel in acidic medium. Ind Eng Chem Res 53:8436–8444
Mistry BM, Patel NS, Sahoo S, Jauhari S (2012) Experimental and quantum chemical studies on corrosion inhibition performance of quinoline derivatives for MS in 1 N HCl. Bull Mater Sci 35:459–469
Ravichandran R, Nanjundan S, Rajendran N (2004) Effect of benzotriazole derivatives on the corrosion and dezincification of brass in neutral chloride solution. J Appl Electrochem 34:1171–1176
Ali SA, El-Shareef AM, Al-Ghandi RF, Saeed MT (2005) The isoxazolidines the effects of a steric factor and hydrophobic chain length on the corrosion inhibition of mild steel in acidic medium. Corr Sci 47:2659–2678
Jayaperumal D (2010) Effects of alcohol-based inhibitors of corrosion of mild steel in hydrochloric acid. Mater Chem Phys 119:478–484
Shanbhag AV, Venkatesha TV, Praveen BM, Abd Hamid SB (2012) Inhibition effect of chloroquinolines on corrosion of mild steel in hydrochloric acid solution. J Iron Steel Res Int 21:804–808
Bentiss F, Lebrini M, Vezin H, Chai F, Traisnel M, Lagrenee M (2009) Enhanced corrosion resistance of carbon steel in the normal sulfuric acid medium by some macrocyclic polyether compounds containing a 1,3,4-thiadiazole moiety: AC impedance and computational studies. Corr Sci 51:2165–2173
Juttner K (1990) Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces. Electrochim Acta 35:1501–1508
Lopez DA, Simison SN, De Sanchez SR (2005) Inhibitors performance in CO2 corrosion EIS studies on the interaction between their molecular structure and steel microstructure. Corr Sci 47:735–755
Bentiss F, Lebrini M, Lagrenee M (2005) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis(n-thienyl)-1,3,4-thiadiazoles/hydrochloric acid system. Corr Sci 47:2915–2931
Khaled KF, Hackerman N (2003) Investigation of the inhibitive effect of ortho-substituted anilines on corrosion of iron in 1 M HCl solutions. Electrochim Acta 48:2715–2723
Abdel Rehim SS, Hazzazi OA, Amin MA, Khaled KF (2008) On the corrosion inhibition of low carbon steel in concentrated sulfuric acid solutions. Part I: chemical and electrochemical (AC and DC) studies. Corr Sci 50:2258–2271
Ferreira ES, Giancomlli C, Giacomlli FC, Spinelli A (2004) Evaluation of the inhibitor effect of l-ascorbic acid on the corrosion of mild steel. Mater Chem Phys 83:129–134
Elyn Amira WAW, Rahim AA, Osman H, Awang K, Bothiraja P (2011) Corrosion inhibition of mild steel in 1 M HCl solution by Xylopia ferruginea leaves from different extract and partitions. Int J Electrochem Sci 6:2998–3016
Muthukrishnan P, Jeyaprabha B, Prakash P (2014) Mild steel corrosion inhibition by aqueous extract of Hyptis suaveolens leaves. Int J Ind Chem 5:5
Qu Q, Jiang SA, Bai W, Li L (2007) Effect of ethylene diamine tetraacetic acid disodium on the corrosion of cold rolled steel in the presence of benzotriazole in hydrochloric acid. Electrochim Acta 52:6811–6820
Karthikaiselvi R, Subhashini S (2014) Study of adsorption properties and inhibition of mild steel corrosion in hydrochloric acid media by water soluble composite poly (vinyl alcohol-o-methoxy aniline. J Assoc Arab Univ Basic Appl Sci 16:74–82
Badiea AM, Mohana KN (2009) Effect of temperature and fluid velocity on corrosion mechanism of low carbon steel in the presence of 2-hydrazino-4, 7-dimethylbenzothiazole in an industrial water medium. Corr Sci 51:2231–2241
Fouda AS, Heakal FE, Radwan MS (2009) Role of some thiadiazole derivatives as inhibitors of the corrosion of C-steel in 1M H2SO4. J Appl Electrochem 39:391–402
Anejjar A, Salghi R, Zarrouk A, Benali O, Zarrok H, Hammouti B, Ebenso EE (2014) Inhibition of carbon steel corrosion in 1 M HCl medium by potassium thiocyanate. J Assoc Arab Univ Basic Appl Sci 15:21–27
Tao Z, Zhang S, Li W, Hou B (2011) Adsorption and inhibitory mechanism of 1H-1,2,4-triazol-l-yl-methyl-2-(4-chlorophenoxy) acetate on corrosion of mild steel in acidic solution. Ind Eng Chem Res 50:6082–6088
Prasanna BM, Praveen BM, Hebbar Narayana, Venkatesha TV (2015) Anticorrosion potential of hydralazine for corrosion of mild steel in 1 M hydrochloric acid solution. J Fundam Appl Sci 7:222–243
Bammou L, Belkhaouda M, Salghi R, Benali O, Zarrouk A, Zarrok H, Hammouti B (2011) Corrosion inhibition of steel in sulfuric acidic solution by the Chenopodium ambrosioides extracts. J Assoc Arab Univ Basic Appl Sci 16:83–90
Acknowledgments
Authors are thankful to Vision Group on Science and Technology (VGST) Government of Karnataka for providing financial assistance under CISEE scheme (Ref No: GRD 313/ dated 01/01/2015). Authors are also grateful to Srinivas School of Engineering, Mukka. For providing laboratory facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Prasanna, B.M., Praveen, B.M., Hebbar, N. et al. Inhibition study of mild steel corrosion in 1 M hydrochloric acid solution by 2-chloro 3-formyl quinoline. Int J Ind Chem 7, 9–19 (2016). https://doi.org/10.1007/s40090-015-0064-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-015-0064-6