Abstract
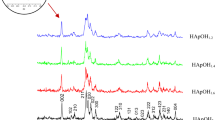
Carbonated apatite is the inorganic component of natural bone while the carbonate ion in the structure influences biological activities and osteoconductivity. However, thermal stability of carbonate apatite is a major importance since thermal stability of carbonate apatite is a function of carbonate content presented in the structure and heat treatment atmosphere. This research work investigates the effects of different carbonate contents on carbonate substitution, thermal stability, physical and mechanical properties of carbonate apatite synthesized by a precipitation method. The results indicated that the carbonate content influenced the properties of carbonated apatite powders. High carbonate substitution (10-11 wt.%) promoted the decomposition of carbonated apatite after heat treatment at 900 °C while a low amount of carbonate substitution (approx. 2 wt.%) resulted in the relocation of carbonate group from phosphate site to hydroxyl site without decomposition. It also demonstrated that a high carbonate amount resulted in a superior mechanical performance. Dense carbonated apatite with high carbonate content has achieved a relative density of 97% and a maximum fracture toughness of 1.4 MPa•m1/2. In the contrary, low carbonate content resulted in a compact with high porosity of about 40% and a low toughness value of 0.35 MPa·m1/2 when treatment at 900 °C.









Similar content being viewed by others
References
M. Vallet-Regi and J.M. González-Calbet, Calcium Phosphates as Substitution of Bone Tissues, Prog. Solid State Chem., 2004, 32(1–2), p 1–31. https://doi.org/10.1016/j.progsolidstchem.2004.07.001
K. Fujisawa, K. Akita, N. Fukuda, K. Kamada, T. Kudoh, G. Ohe and Y. Miyamoto, Compositional and Histological Comparison of Carbonate Apatite Fabricated by Dissolution-Precipitation Reaction and Bio-Oss®, J. Mater. Sci. Mater. Med., 2018, 29(8), p 1–11. https://doi.org/10.1007/s10856-018-6129-2
K. Kuroda and M. Okido, Hydroxyapatite Coating of Titanium Implants Using Hydroprocessing and Evaluation of Their Osteoconductivity, Bioinorganic Chem. Appl., 2012, 2012, p 1–7. https://doi.org/10.1155/2012/730693
Y. Doi, H. Iwanaga, T. Shibutani, Y. Moriwaki and Y. Iwayama, Osteoclastic Responses to Various Calcium Phosphates in Cell Cultures, J. Biomed. Mater. Res., 1999, 47(3), p 424–433. https://doi.org/10.1002/(SICI)1097-4636(19991205)47:3%3c424::AID-JBM19%3e3.0.CO;2-0
E. Landi, G. Celotti, G. Logroscino and A. Tampieri, Carbonated Hydroxyapatite as Bone Substitute, J. Eur. Ceram. Soc., 2003, 23(15), p 2931–2937. https://doi.org/10.1016/S0955-2219(03)00304-2
K. Ishikawa, Bone Substitute Fabrication Based on Dissolution-Precipitation Reactions, Materials, 2010, 3(2), p 1138–1155. https://doi.org/10.3390/ma3021138
H Nagai, M Kobayashi-Fujioka, K Fujisawa, G Ohe, N Takamaru, K Hara, D Uchida, T Tamatani, K Ishikawa, Y Miyamoto, Effects of Low Crystalline Carbonate Apatite on Proliferation and Osteoblastic Differentiation of Human Bone Marrow Cells J. Mater. Sci. Mater. Med. 26 2 (2015) https://doi.org/10.1007/s10856-015-5431-5
G. Spence, N. Patel, R. Brooks, W. Bonfield and N. Rushton, Osteoclastogenesis on Hydroxyapatite Ceramics: The Effect of Carbonate Substitution, J Biomed. Mater. Res. A, 2010, 92(4), p 1292–300.
A.A. Baig, J.L. Fox, J. Hsu, Z. Wang, M. Otsuka, W.I. Higuchi and R. Z. LeGeros, Effect of Carbonate Content and Crystallinity on the Metastable Equilibrium Solubility Behavior of Carbonated Apatites, J. Coll. Interface Sci., 1996, 179(2), p 608–617. https://doi.org/10.1006/jcis.1996.0255
Z. Zyman and M. Tkachenko, CO2 Gas-Activated Sintering of Carbonated Hydroxyapatites, J. Eur. Ceram. Soc., 2011, 31(3), p 241–248. https://doi.org/10.1016/j.jeurceramsoc.2010.09.005
Q. Liu, J.P. Matinlinna, Z. Chen, C. Ning, G. Ni, H. Pan and B.W. Darvell, Effect of Thermal Treatment on Carbonated Hydroxyapatite: Morphology, Composition, Crystal Characteristics and Solubility, Ceram. Int., 2015, 41(5), p 6149–6157. https://doi.org/10.1016/j.ceramint.2014.11.062
A. Ślósarczyk, Z. Paszkiewicz and A. Zima, The Effect of Phosphate Source on the Sintering of Carbonate Substituted Hydroxyapatite, Ceram. Int., 2010, 36(2), p 577–582. https://doi.org/10.1016/j.ceramint.2009.09.032
E. Landi, A. Tampieri, G. Celotti, L. Vichi and M. Sandri, Influence of Synthesis and Sintering Parameters on the Characteristics of Carbonate Apatite, Biomaterials, 2004, 25(10), p 1763–1770. https://doi.org/10.1016/j.biomaterials.2003.08.026
J. Barralet, J.C. Knowles, S. Best and W. Bonfield, Thermal Decomposition of Synthesised Carbonate Hydroxyapatite, J. Mater. Sci. Mater. Med., 2002, 13(6), p 529–533.
M. Safarzadeh, Chin Fei Chee, S. Ramesh and M.N. Ahmad Fauzi, Effect of Sintering Temperature on the Morphology, Crystallinity and Mechanical Properties of Carbonated Hydroxyapatite (CHA), Ceram. Int., 2020, 46(17), p 26784–26789. https://doi.org/10.1016/j.ceramint.2020.07.153
I.R. Gibson and W. Bonfield, Novel Synthesis and Characterization of an AB-Type Carbonate-Substituted Hydroxyapatite, J. Biomed. Mater. Res., 2002, 59(4), p 697–708. https://doi.org/10.1002/jbm.10044
J. Barralet, J.C. Knowles, S. Best and W. Bonfield, Thermal Decomposition of Synthesised Carbonate Hydroxyapatite, J. Mater. Sci. Mater. Med., 2002, 13(6), p 529–533. https://doi.org/10.1023/a:1015175108668
A.G. Evans and E.A. Charles, Fracture Toughness Determinations by Indentation, J. Am. Ceram. Soc., 1976, 59(7–8), p 371–372. https://doi.org/10.1111/j.1151-2916.1976.tb10991.x
I.Y. Pieters, N.M.F. Van den Vreken, H.A. Declercq, M.J. Cornelissen and R.M.H. Verbeeck, Carbonated Apatites Obtained by the Hydrolysis of Monetite: Influence of Carbonate Content on Adhesion and Proliferation of MC3T3-E1 Osteoblastic Cells, Acta Biomater., 2010, 6(4), p 1561–1568. https://doi.org/10.1016/j.actbio.2009.11.002
J.P. Lafon, E. Champion and D. Bernache-Assollant, Processing of AB-Type Carbonated Hydroxyapatite Ca10−x(PO4)6−x(CO3)x(OH)2−x−2y(CO3)y Ceramics with Controlled Composition, J. Eur. Ceram. Soc., 2008, 28(1), p 139–147. https://doi.org/10.1016/j.jeurceramsoc.2007.06.009
Y.D.T. Koda, N. Wakamatsu, T. Goto, H. Kamemizu, Y. Moriwaki, M. Adachi and Y. Suwa, Influence of Carbonate on Sintering of Apatites, J. Dent. Res., 1993, 72(9), p 1279–1284.
J.C. Merry, I.R. Gibson, S.M. Best and W. Bonfield, Synthesis and Characterization of Carbonate Hydroxyapatite, J. Mater Sci. Mater. Med., 1998, 9(12), p 779–783.
I.R. Gibson, S.M. Best and W. Bonfield, Chemical Characterization of Silicon-Substituted Hydroxyapatite, J. Biomed. Mater. Res., 1999, 44(4), p 422–428. https://doi.org/10.1002/(sici)1097-4636(19990315)44:4%3c422::aid-jbm8%3e3.0.co;2-#
M. Veiderma, K. Tõnsuaadu, R. Knubovets and M. Peld, Impact of Anionic Substitutions on Apatite Structure and Properties, J. Organometall. Chem., 2005, 690(10), p 2638–2643. https://doi.org/10.1016/j.jorganchem.2004.11.022
LeGeros RZ and JP LeGeros, Calcim Phosphate Bioceramic: Past, Present and Future, in Bioceramic 15, B. Ben-Nissan, D. Sher, and W. Walsh, Editors. 2003, Trans Tech Publication, Sydney. 3–10.
R.Z. LeGeros and J.P. LeGeros, Dense Hydroxyapatite, An Introduction to Bioceramics. L.L. Hench, J. Wilson Ed., WORLD SCIENTIFIC, 1993, p 139–180. https://doi.org/10.1142/9789814317351_0009
X.L. Tang, X.F. Xiao and R.F. Liu, Structural Characterization of Silicon-Substituted Hydroxyapatite Synthesized by a Hydrothermal Method, Mater. Lett., 2005, 59(29–30), p 3841–3846. https://doi.org/10.1016/j.matlet.2005.06.060
T. Tian, D. Jiang, J. Zhang and Q. Lin, Synthesis of Si-Substituted Hydroxyapatite by a Wet Mechanochemical Method, Mater. Sci. Eng. C, 2008, 28(1), p 57–63. https://doi.org/10.1016/j.msec.2007.10.049
J.L. Xu and K.A. Khor, Chemical Analysis of Silica Doped Hydroxyapatite Biomaterials Consolidated by a Spark Plasma Sintering Method, J. Inorg. Biochem., 2007, 101(2), p 187–195. https://doi.org/10.1016/j.jinorgbio.2006.09.030
M. Chandrasekhar Kothapalli, A. Wei and M.T.S. Vasiliev, Influence of Temperature and Concentration on the Sintering Behavior and Mechanical Properties of Hydroxyapatite, Acta Mater., 2004, 52(19), p 5655–5663. https://doi.org/10.1016/j.actamat.2004.08.027
M.R. Saeri, A. Afshar, M. Ghorbani, N. Ehsani and C.C. Sorrell, The Wet Precipitation Process of Hydroxyapatite, Mater. Lett., 2003, 57(24–25), p 4064–4069. https://doi.org/10.1016/s0167-577x(03)00266-0
S.C. Cox, P. Jamshidi, L.M. Grover and K.K. Mallick, Low Temperature Aqueous Precipitation of Needle-like Nanophase Hydroxyapatite, J Mater Sci Mater Med, 2014, 25(1), p 37–46.
J. Lafon, E. Champion, D. Bernache-Assollant, R. Gibert and A. Danna, Thermal Decomposition of Carbonated Calcium Phosphate Apatites, J. Therm. Anal. Calorim., 2003, 72(3), p 1127–1134. https://doi.org/10.1023/a:1025036214044
A. Ślósarczyk, Z. Paszkiewicz and C. Paluszkiewicz, FTIR and XRD Evaluation of Carbonated Hydroxyapatite Powders Synthesised by Wet Methods, J. Mol. Struct., 2005, 744–747, p 657–661.
M. Vignoles, G. Bonel, D. Holcomb and R. Young, Influence of Preparation Conditions on the Composition of Type B Carbonated Hydroxyapatite and on the Localization of the Carbonate Ions, Calcif. Tissue Int., 1988, 43(1), p 33–40. https://doi.org/10.1007/bf02555165
R.Z. LeGeros, Effect of Carbonate on the Lattice Parameters of Apatite, Nature, 1965, 206(4982), p 403–404.
J. Barralet, S. Best and W. Bonfield, Carbonate Substitution in Precipitated Hydroxyapatite: An Investigation Into the Effects of Reaction Temperature and Bicarbonate Ion Concentration, J. Biomed. Mater. Res., 1998, 41(1), p 79–86. https://doi.org/10.1002/(sici)1097-4636(199807)41:1%3c79::aid-jbm10%3e3.0.co;2-c
LeGeros R.Z and J.P LeGeros, Calcium Phosphate Biomaterials Medical Application, in Bioceramics ed by T. Kobuko, T. Nakamura, and F. Miyaji, (Elviser Science: Otsu, Japan, 1996)
S.M. Barinov, J.V. Rau, S.N. Cesaro, J. Ďurišin, I.V. Fadeeva, D. Ferro, L. Medvecky and G. Trionfetti, Carbonate Release from Carbonated Hydroxyapatite in the Wide Temperature Rage, J. Mater. Sci. Mater. Med., 2006, 17(7), p 597–604. https://doi.org/10.1007/s10856-006-9221-y
S. Matsuya, X. Lin, K.-i Udoh, M. Nakagawa, R. Shimogoryo, Y. Terada and K. Ishikawa, Fabrication of Porous Low Crystalline Calcite Block By Carbonation of Calcium Hydroxide Compact, J. Mater. Sci. Mater. Med., 2007, 18(7), p 1361–1367. https://doi.org/10.1007/s10856-007-0123-4
X. Lin, S. Matsuya, M. Nakagawa, Y. Terada and K. Ishikawa, Effect of molding pressure on fabrication of low-crystalline calcite block, J. Mater. Sci. - Mater. Med., 2008, 19(2), p 479–484. https://doi.org/10.1007/s10856-006-0028-7
C. Schiller and M. Epple, Carbonated Calcium Phosphates are Suitable pH-Stabilising Fillers for Biodegradable Polyesters, Biomaterials, 2003, 24(12), p 2037–2043. https://doi.org/10.1016/S0142-9612(02)00634-8
Z. Zyman, D. Rokhmistrov, V. Glushko and I. Ivanov, Thermal Impurity Reactions and Structural Changes in Slightly Carbonated Hydroxyapatite, J. Mater. Sci. Mater. Med., 2009, 20(7), p 1389–1399. https://doi.org/10.1007/s10856-009-3706-4
L.M. Miller, V. Vairavamurthy, M.R. Chance, R. Mendelsohn, E.P. Paschalis, F. Betts and A.L. Boskey, In Situ Analysis of Mineral Content and Crystallinity in Bone Using Infrared Micro-Spectroscopy of the ν4 PO43-Vibration, Biochimica et Biophysica Acta (BBA) Gen. Subj., 2001, 1527(1–2), p 11–19. https://doi.org/10.1016/S0304-4165(01)00093-9
C. Ortali, I. Julien, C. Drouet and E. Champion, Influence of Carbonation on the Low-Temperature Consolidation by Spark Plasma Sintering of Carbonated Calcium Phosphate Bioceramics, Ceram. Int., 2020, 46(5), p 5799–5810. https://doi.org/10.1016/j.ceramint.2019.11.030
J. Wang and L.L. Shaw, Nanocrystalline Hydroxyapatite with Simultaneous Enhancements in Hardness and Toughness, Biomaterials, 2009, 30(34), p 6565–6572. https://doi.org/10.1016/j.biomaterials.2009.08.048
P. Kamalanathan, S. Ramesh, L.T. Bang, A. Niakan, C.Y. Tan, J. Purbolaksono, H. Chandran and W.D. Teng, Synthesis and Sintering of Hydroxyapatite Derived from Eggshells as a Calcium Precursor, Ceram. Int., 2014, 40(10), p 16349–16359. https://doi.org/10.1016/j.ceramint.2014.07.074
D. Veljovic, E. Palcevskis, I. Zalite, R. Petrovic and D. Janackovic, Two-Step Microwave Sintering-A Promising Technique for the Processing of Nanostructured Bioceramics, Mater. Lett., 2013, 93, p 251–253. https://doi.org/10.1016/j.matlet.2012.11.095
S. Ramesh, C.Y. Tan, R. Tolouei, M. Amiriyan, J. Purbolaksono, I. Sopyan and W.D. Teng, Sintering Behavior of Hydroxyapatite Prepared from Different Routes, Mater. Des., 2012, 34, p 148–154. https://doi.org/10.1016/j.matdes.2011.08.011
M. Rahaman, Sintering of ceramics, CRC Press, Boca Raton, 2008.
Acknowledgments
This research work is supported by National Foundation for Science and Technology Development (Nafosted), Vietnam [Grant No.104.03-2020.36].
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thang, L.H., Bang, L.T., Long, B.D. et al. Effect of Carbonate Contents on the Thermal Stability and Mechanical Properties of Carbonated Apatite Artificial Bone Substitute. J. of Materi Eng and Perform 32, 1006–1016 (2023). https://doi.org/10.1007/s11665-022-07169-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-022-07169-6