Abstract
Wetting angles of Sn-8.8Zn and Sn-8.8Zn-xIn alloys (x = 0.5, 1.0, 1.5 wt%) were studied with the sessile drop method. Wetting tests were carried out for 900 s in the presence of ORM0 flux at 493, 523, and 573 K on copper and at 523 K on nickel substrates, respectively. It was found that the addition of In to Sn-8.8Zn alloy improves its wetting on both substrates by reducing the value of apparent wetting angle. Also, with increasing temperature a decrease of wetting angle on copper is observed in the case of 0 and 1.5 wt% of In alloys. Solidified solder-substrate couples were cross-sectioned and examined with scanning electron microscopy coupled with electron dispersive X-ray analysis. Interlayers were found at the interface of solders with copper and nickel, and their compositions are close to Cu5Zn8 and Ni5Zn21 intermetallics, respectively. However, in the case of Sn-8.8Zn-1.5/Ni couple small scallops are observed instead of continuous interlayer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among Pb-free alternatives to traditional solders, Sn-8.8Zn eutectic alloy has the closest melting point (m.p. ~471 K) to the Sn-40Pb solder (m.p. ~456 K), much lower compared to broadly used SAC solder (m.p. ~490 K). This is quite important as the low soldering temperature reduces possible heat damage to joined elements. Also the temperature range could be broadened in the case of step-soldering process. One important factor in favor of Sn-Zn solder is its low price per mass unit, as a result of low price of Zn. In the long term, price of zinc is roughly 10% of price of tin, which is also a few orders lower than price of silver (Ref 1). As pointed out elsewhere (Ref 2, 3), the main disadvantages of Sn-Zn solder are susceptibility to oxidation and a relatively poor wettability compared to other Pb-free alternatives. Moreover, due to formation of brittle intermetallic compounds (IMC), such as Cu5Zn8 and Sn6Cu5, at the Sn-Zn solder-Cu substrate interface the joint strength is weaker compared to other solders (Ref 2). Several papers discussed wettability, oxidation resistance, melting, mechanical properties, and microstructures of Sn-Zn based solders and interfaces with Cu and Ni substrates, a great number of them was reviewed by Zhang et al. (Ref 4). McCormack et al. (Ref 5) were the first to propose Sn-Zn-In solder. They found the melting temperature of Sn-9Zn-5In (wt%) 10 K lower than 471 K of Sn-9Zn and wetting behavior (wetting force) improved compared to Sn-9Zn solder, yet pointed out that further testing of this systems’ properties is necessary. They also pointed out the wetting force of Sn-9Zn-5In solder strongly depends on the flux used. This was confirmed by Yu et al. (Ref 6), who identified Cu5Zn8 intermetallic at the solder/Cu substrate interface. Takemoto and Miyazaki (Ref 7) used wetting balance technique to study the effect of indium in the range of 5-25 wt% on wetting of Sn-Zn-In alloys on copper. To the best of this authors’ knowledge, there are no published works on wetting of Sn-Zn-In solders on nickel substrates. Phase diagram and thermodynamic properties of ternary Sn-Zn-In system were evaluated by Cui et al. (Ref 8). The calculated thermochemical quantities as well as vertical sections of the phase diagram well agree with experimental data in most cases. Their findings suggest that the optimum indium concentration in the Sn-Zn-In alloys should not exceed 5 wt%, since in this composition range a simple eutectic microstructure (without indium-rich gamma phase) can be obtained. In the light of the above, the aim of this work is to study the effect of small In content (0.5-1.5 wt%) in the Sn-Zn-In solders on their wetting behavior on Cu and Ni substrates, and the microstructure of solder/substrate interface, in the presence of organic moderately active flux (ORM0—according to IPC J-STD-004 standard).
Experimental
Sn-8.8Zn (eutectic) and three solders based on it containing small addition of indium: 0.5; 1.0; 1.5 wt%, respectively, were used for a study of wetting on Cu (99.9%) and Ni (99.5%) substrates with the sessile drop (SD) method. Solders were prepared by melting pure metals (99.999%) in graphite crucibles, under Ar (99.9992%) protective atmosphere to avoid oxidation. The liquid alloys were sucked into quartz capillaries of 2 mm uniform inner diameter and after solidification the obtained rods were cut into 3 mm high pieces. Both solders and substrates (25 × 20 × 0.2 mm) prior the tests were degreased with acetone. The wetting tests were carried out at 523 K on nickel substrates and three temperatures: 493, 523, 573 K on copper substrates, with the setup described in detail in (Ref 9). The setup enables quick transfer of the sample to the already heated furnace, so the heating rate is very fast, as well as quick transfer out after the designated time of the test. During the tests, organic moderately active flux ORM0 was applied, and all of the samples were kept in the test temperature for 900 s. The reported wetting angles are the average of three independent experiments. After wetting tests, selected solidified solder/substrate couples were cut perpendicular to the plane of the interface, mounted in conductive resin and polished for microstructural characterization. The microstructural and EDS analysis was performed with FEI E-SEM XL30 system, at 20 kV and WD 10 mm, with the use of the standardless Analysis EDAX System based on Genesis 2000 software.
Results and Discussion
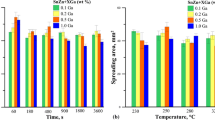
The results of wetting tests on copper and nickel substrates are shown in Fig. 1(a) and (b), respectively. Error bars imposed in Fig. 1 denote standard deviation of the experimental data. One could see that for Sn-8.8Zn and Sn-8.7Zn-1.5In alloys on copper substrate there is a tendency of decreasing of the wetting angle with increasing temperature. For Sn-8.8Zn-0.5In and Sn-8.8Zn-1.0In alloys no such tendency can be observed. As far as wetting angle dependence on composition is concerned, on Cu substrate only at 493 K the wetting angle decreases with increasing concentration of In. At 523 K the wetting angles of In-containing alloys are nearly the same, whereas at 573 K wetting angle of alloy containing 1.0 wt% of In is higher. On Ni substrate the wetting angle decreases with In concentration increasing from 0 to 1 wt%, but the wetting angle of 1.5 wt% In alloy is slightly higher than that of 1 wt% In alloy. Taking into account the uncertainty of wetting angle measurement (Fig. 1b), however, it can be assumed that the wetting angle of Sn-Zn-In alloys on Ni substrate is nearly the same. Wetting angles of alloys containing In are lower than those of Sn8.8Zn alloy, both on copper and nickel substrates, although the wetting angles on nickel are generally higher than wetting angles of respective alloys determined on copper at 523 K. The wetting angles on copper are in the range 34°-46.5° and those on nickel in the range 39.5°-49.5°. According to the classification of wetting by Klein-Wassink (Ref 10), the wetting angles in the range 30°-40° indicate good wetting and the wetting angles in the range 40°-50° indicates sufficient wetting. As pointed before, authors (Ref 5-7) studied wetting of Sn-Zn-In solders on copper with wetting balance technique. Of them, only Takemoto used their results to calculate the wetting angles. The wetting angle of 58.5° that they reported for Sn-7.9Zn-5In alloy at 503 K is much higher than our results. This can not only be attributed to the differences in composition but to the differences between the experimental methods and different flux used. The results of wetting angle measurements are strongly dependent on the flux. This is clearly seen when comparing the results obtained with the same method at the same temperature. For example, Cheng at al. (Ref 11) and Huang et al. (Ref 12) measured the wetting angle of Sn-9Zn alloy on copper at 523 K and found it to be 54° and 39.5°, respectively. Chan et al. (Ref 13), with the SD method in vacuum at 523 K, studied wetting of Sn-9Zn alloys on copper and nickel, and reported wetting angles of 38° and 35°, respectively.
Figure 2(a) and (b) presents the microstructure of Sn-8.8Zn/Cu and Sn-8.7Zn-1.5In/Cu cross-sectioned samples, whereas Fig. 3(a) and (b) presents the microstructure of Sn-8.8Zn/Ni and Sn-8.7Zn-1.5In/Ni couples, respectively, at 523 K. Dashed lines on the right side of each micrograph mark the edge of a substrate. The results of EDS analysis in the areas marked in Fig. 2 and 3 are collected in Table 1. In the case of samples presented in Fig. 2, at the solder-substrate interface the interlayer containing zinc and copper is observed. The EDS analysis of the interlayer indicates that in both cases the interlayer composition is about 35 wt% of Cu and 65 wt% of Zn. This observation is in agreement with the data reported by earlier authors (Ref 13, 14). With the X-ray diffraction, Yu et al. (Ref 6) confirmed the interlayer to be Cu5Zn8. In the Sn-8.8Zn solder, near the interlayer reduction of zinc concentration to 4.2 wt% is observed, compared to nominal composition of the solder. This is also apparent in the case of Sn-8.7Zn-1.5In/Cu couple, where near the interface there is 4.5 wt% of Zn in the solder.
Contrary to the layer observed on copper (Fig. 2a), the interlayer between Sn-8.8Zn solder and nickel (Fig. 3a) substrate is thin, with the average thickness of 5 μm. The interlayer composition is Zn with 17.2 wt% of Ni and 4.5 wt% of Sn, which is close to Zn-15.8Ni-2.6Sn found by Chou et al. (Ref 14), who identified the interlayer as γ-Ni5Zn21 phase. Chang et al. (Ref 15) studied isothermal cross-sections in the Sn-Ni-Zn ternary and found that maximum solubility of Sn in γ-phase is 4.2 and 5.5 wt% at 473 and 1073 K, respectively. The interfacial reactions between Sn-Zn alloys and Ni substrate were studied by Zhu et al. (Ref 16), similarly to an earlier work of Chan et al. (Ref 13) they found that a single and relatively flat on both sides interlayer is formed at the liquid Sn-Zn/Ni substrate interface and found its composition close to γ-Ni5Zn21 phase. Although, according to phase diagrams a few other phases from Ni-Zn and Ni-Sn systems are stable at this temperature, the formation of γ-Ni5Zn21 layer is possible thanks to its fast nucleation and growth (Ref 16). Chou et al. (Ref 14) and Zhu et al. (Ref 16) studied growth kinetics of the γ-Ni5Zn21 phase in the Cu-9Zn/Ni couples and found it to be strongly time dependent. A comparison of Sn-8.8Zn/Ni and Sn-8.7Zn-1.5In/Ni interfaces, presented in Fig. 3(a) and (b) after 900 s of wetting at 523 K, indicates that in the latter case instead of continuous interlayer only small scallops can be found. According to EDS analysis composition of scallops in Fig. 3(b) is Zn with 15.6 wt% of Ni, 7.7 wt% of Sn, and 0.8 wt% of In. This is close to the composition of the inlayer in Fig. 3(a); therefore, it can be assumed it is also γ-Ni5Zn21 phase. The height of scallops in Fig. 3(b) does not exceed 3 μm and is smaller than the maximum thickness of layers observed after the same time of wetting (900 s) in the case of Sn-8.8Zn/Ni (10 μm), Sn-8.7Zn-1.5In/Cu (13 μm), and Sn-8.8Zn/Cu (18 μm) couples, respectively. Based on the above, it can be speculated that the addition of indium to Sn-8.8Zn alloy inhibits the growth of γ -phase intermetallic on nickel substrate. However, in order to confirm this further studies are needed.
Conclusion
Wetting behavior of Sn-Zn and Sn-8.8Zn-(xIn) (x = 0.5, 1.0, 1.5 wt%) alloys on copper and nickel substrates, respectively, was studied with the SD method in the presence of ORM0 flux. It was found that the examined alloys exhibit good, or at least sufficient wetting on both types of substrates, as the wetting angles are in the range 34°-49.5°. Generally, both on Cu and Ni substrates wetting angles of alloys containing In are lower than wetting angles of Sn-8.8Zn alloy. With increasing temperature a decrease of wetting angle on Cu is observed for Sn-8.8Zn and Sn-8.7Zn-1.5In alloys, but not for Sn-8.7Zn-1.0In and Sn-8.8Zn-0.5In alloys. The microstructure analysis of solder-copper couples indicates continuous Cu5Zn8 layer at the interface. In the case of solder-nickel couples Ni5Zn21 intermetallic is formed at the interface. However, on Ni substrate for Sn-8.7Zn-1.5In solder, the Ni5Zn21 intermetallic is discontinuous and thin compared to the layer formed by Sn-8.8Zn solder. Based on the present results and literature studies of the Ni5Zn21 growth kinetics in Sn-9Zn/Ni couples it can be speculated, that the growth of the Ni5Zn21 in Sn-8.8Zn-1.5In/Ni couples is inhibited by indium.
References
S.A. Mucklejohn, D.R. Anderson, N. Hoo, A. Kondentsov, A. Kroupa, I. Plotog, Z. Toth, and A. Watson, Protocol for the Evaluation of Alloys to be Used as Replacements for High Temperature Lead-Containing Solders in Products & Industrial Processes, COST Action MP0602 Advanced Solder Materials for High Temperature Application (HiSOLD), June 22-24, 2011 (Brno)
T. Ichitsubo, E. Matsubara, K. Fujiwara, M. Yamaguchi, H. Irie, S. Kumamoto, and T. Anada, Control of Compound Forming Reaction at the Interface Between SnZn Solder and Cu Substrate, J. Alloys Compd., 2005, 392(1–2), p 200–205
K. Suganuma and K.S. Kim, Sn-Zn Low Temperature Solder, J. Mater. Sci.: Mater. Electron., 2007, 18(1–3), p 121–127
L. Zhang, S. Xue, L. Gao, Z. Sheng, and H. Ye, Development of Sn-Zn Lead-Free Solders Bearing Alloying Elements, J. Mater. Sci.: Mater. Electron., 2010, 21(1), p 1–15
M. McCormack, S. Jin, H.S. Chen, and D.A. Machusak, New Lead-Free Sn-Zn-ln Solder Alloys, J. Electron. Mater., 1994, 23(7), p 687–690
S. Yu, C. Liao, M. Hon, and M. Wang, The Effects of Flux on the Wetting Characteristics of Near-Eutectic Sn-Zn-In Solder on Cu Substrate, J. Mater. Sci., 2000, 35(17), p 4217–4224
T. Takemoto and M. Miazaki, Effect of Excess Temperature Above Liquidus of Lead-Free Solders on Wetting Time in Wetting Balance Test, Mater. Trans., 2001, 42(5), p 745–750
Y. Cui, X.J. Liu, I. Ohnuma, R. Kainuma, H. Ohtani, and K. Ishida, Thermodynamic Calculation of the In-Sn-Zn Ternary System, J. Alloys Compd., 2001, 320(2), p 234–241
P. Fima, W. Gasior, A. Sypien, and Z. Moser, Wetting of Cu by Bi-Ag Based Alloys with Sn and Zn Additions, J. Mater. Sci., 2010, 45(16), p 4339–4344
R.J. Klein-Wassink, Soldering in Electronics, 2nd ed., Electrochemical Publications LTD, Isle of Man, 1989
S.C. Cheng and K.L. Lin, The Thermal Property of Lead-Free Sn-8.55Zn-1Ag-XAl Solder Alloys and Their Wetting Interaction with Cu, J. Electron. Mater., 2002, 31(9), p 940–945
C.W. Huang and K.L. Lin, Wetting Properties of and Interfacial Reactions in Lead-Free Sn-Zn Based Solders on Cu and Cu Plated with an Electroless Ni-P/Au Layer, Mater. Trans., 2004, 45(2), p 588–594
Y.C. Chan, M.Y. Chiu, and T.H. Chuang, Intermetallic Compounds Formed During the Soldering Reactions of Eutectic Sn-9Zn with Cu and Ni Substrates, Z. Metallkd., 2002, 93(2), p 95–98
C. Chou, S.W. Chen, and Y. Chang, Interfacial Reactions in the Sn-9Zn-(xCu)/Cu and Sn-9Zn-(xCu)/Ni Couples, J. Mater. Res., 2006, 21(7), p 1849–1856
J. Chang, S.-K. Seo, and H.M. Lee, Phase Equilibria in the Sn-Ni-Zn Ternary System: Isothermal Sections at 200 °C, 500 °C, and 800 °C, J. Electron. Mater., 2010, 39(12), p 2643–2652
W. Zhu, H. Liu, J. Wang, G. Ma, and Z. Jin, Interfacial Reactions Between Sn-Zn Alloys and Ni Substrates, J. Electron. Mater., 2010, 39(2), p 209–214
Acknowledgments
This work was financed under the framework of the project POIG.01.01.02-00-015/09, co-funded by the European Regional Development Fund (ERDF) and the Government of Poland under the Innovative Economy Program
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Fima, P., Gancarz, T., Pstruś, J. et al. Wetting of Sn-Zn-xIn (x = 0.5, 1.0, 1.5 wt%) Alloys on Cu and Ni Substrates. J. of Materi Eng and Perform 21, 595–598 (2012). https://doi.org/10.1007/s11665-012-0124-4
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-012-0124-4