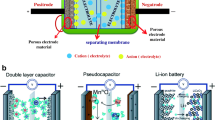
Abstract
Global carbon reduction targets can be facilitated via energy storage enhancements. Energy derived from solar and wind sources requires effective storage to guarantee supply consistency due to the characteristic changeability of its sources. Supercapacitors (SCs), also known as electrochemical capacitors, have been identified as a key part of solving the problem. In addition, SCs can provide solutions to charging electric vehicles much faster than is possible using lithium-ion batteries. Nevertheless, further research into high-performance supercapacitor development is urgently needed to enable their use for effective large electricity storage. In general, energy utilization will subsequently depend on consumers/industries that are generating, storing and utilizing energy more effectively, with SCs being identified as one of the emerging technologies for intermittent energy storage, harvesting and high-power delivery. In this review, we have highlighted the historical information concerning the evolution of supercapacitor technology and its application as an energy storage device. A detailed account of the device’s electrode materials/electrolytes, processes, designs, and various applications is discussed. The primary characteristics of the energy storage system, such as capacitance/capacity, operating temperature, energy/power density, operating potential, kinetic storage mechanism, cycling lifetime, self-discharge, voltage holding/floating test, and the makeup of the electrode materials, are also briefly discussed. In addition, based on the current research scenario, the potential, challenges, and development patterns for SCs are summarized.






















Similar content being viewed by others
References
B.E. Conway, Electrochemical Supercapacitors (Boston: Springer, 1999).
F. Béguin, V. Presser, A. Balducci, and E. Frackowiak, Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 26, 2219–2251 (2014).
C.A. McGill, A brief History of A Brief History. Pop. Sci. 235, 70–72 (2010).
Miller, A brief history of supercapacitors, Batter. + Energy. ISSN: 521452 (2007) 61–78.
K.O. Oyedotun, M.J. Madito, D.Y. Momodu, A.A. Mirghni, T.M. Masikhwa, and N. Manyala, Synthesis of Ternary NiCo-MnO2 Nanocomposite and its Application as a Novel High Energy Supercapattery Device. Chem. Eng. J. 335, 416–433 (2018).
N. Choudhary, C. Li, J. Moore, N. Nagaiah, L. Zhai, Y. Jung, and J. Thomas, Asymmetric Supercapacitor Electrodes and Devices. Adv. Mater. 29, 1605336 (2017).
G. Xiong, A. Kundu, and T.S. Fisher, Thermal Management in Electrochemical Energy Storage Systems. Springerbriefs Appl. Sci. Technol. 25, 1–10 (2015).
D. Institute of Fuel (Great Britain), Elsevier Science Ltd., Fuel and energy abstracts., [Published on behalf of the Institute of Fuel by IPC Science and Technology Press], (1995)
T.L. Floyd and D. Buchla, Electronics Fundamentals: Circuits, Devices & Applications, 2009th ed., (NJ: Prentice Hall Press, 2009).
E. Frackowiak and F. Beguin, Carbon Materials for the Electrochemical Storage of Energy in Capacitors. Carbon 39, 937–950 (2001).
B.E. Conway, Electrochemical Supercapacitors - Scientific Fundamentals (Berlin: Springer, 1999).
A. Lahyani, P. Venet, A. Guermazi, and A. Troudi, Battery/Supercapacitors Combination in Uninterruptible Power Supply (UPS). IEEE Trans. Power Electron. 28, 1509–1522 (2013).
Y.B. Tan and J.M. Lee, Graphene for Supercapacitor Applications. J. Mater. Chem. A 1, 14814–14843 (2013).
F. Béguin, E. Raymundo-Piñero, and E. Frackowiak, Electrical Double-Layer Capacitors and Pseudocapacitors (Cambridge: CRC Press, 2009).
K.O. Oyedotun, Synthesis and characterization of carbon-based nanostructured material electrodes for designing novel hybrid supercapacitors, PhD diss., University of Pretoria (2018). http://hdl.handle.net/2263/67866
A. Bard, L. Faulkner, J. Leddy, and C. Zoski, Electrochemical Methods: Fundamentals and Applications, Vol. 2 (NY: Wiley, 1980).
L.L. Zhang and X.S. Zhao, Carbon-Based Materials as Supercapacitor Electrodes. Chem. Soc. Rev. 38, 2520 (2009).
A.G.G. Pandolfo and A.F.F. Hollenkamp, Carbon Properties and Their Role in Supercapacitors. J. Power Sources. 157, 11–27 (2006).
M.A. Brown, Z. Abbas, A. Kleibert, R.G. Green, A. Goel, S. May, and T.M. Squires, Determination of Surface Potential and Electrical Double-Layer Structure at the Aqueous Electrolyte-Nanoparticle Interface. Phys. Rev. X. 6, 011007 (2016).
Z. Wang, Y. Zhong, C. Wei, L. Jiang, and H. Liu, Review—Metal-Organic Framework-Based Supercapacitors. J. Electrochem. Soc. 169, 010516 (2022).
P. Simon and Y. Gogotsi, Materials for Electrochemical Capacitors. Nat. Mater. 7, 845–854 (2008).
D.P. Dubal, O. Ayyad, V. Ruiz, and P. Gómez-Romero, Hybrid Energy Storage: The MERGING of Battery and Supercapacitor Chemistries. Chem. Soc. Rev. 44, 1777–1790 (2015).
G.G. Amatucci, F. Badway, A. Du Pasquier, and T. Zheng, An Asymmetric Hybrid Nonaqueous Energy Storage Cell. J. Electrochem. Soc. 148, A930 (2001).
P. Forouzandeh, V. Kumaravel, and S.C. Pillai, Electrode Materials for Supercapacitors: A Review of Recent Advances. Catalysts 10, 1–73 (2020).
V. Khomenko, E. Raymundo-Piñero, and F. Béguin, High-Energy Density Graphite/AC Capacitor in Organic Electrolyte. J. Power Sources. 177, 643–651 (2008).
T. Brousse, M. Toupin, and D. Bélanger, A Hybrid Activated Carbon-Manganese Dioxide Capacitor Using A Mild Aqueous Electrolyte. J. Electrochem. Soc. 151, A614 (2004).
A.J. Chmiola, G. Yushin, Y. Gogotsi, C. Portet, P. Simon, and P.L. Taberna, Anomalous Capacitance Less Than INCREASE in Carbon at Pore Sizes. Science 313, 1760–1763 (2015).
X. Zhang, X. Wang, L. Jiang, H. Wu, C. Wu, and J. Su, Effect of Aqueous Electrolytes on the Electrochemical Behaviors of Supercapacitors Based on Hierarchically Porous Carbons. J. Power Sources. 216, 290–296 (2012).
Q. Pan, W. Tu, L. Ding, and G. Mi, Characteristics of Electric Double Layer in Different Aqueous Electrolyte Solutions for Supercapacitors. Wuhan Univ. J Nat. Sci. 17, 200–204 (2012).
K. Fic, G. Lota, and E. Frackowiak, Electrochemical Properties of Supercapacitors Operating in Aqueous Electrolyte with Surfactants. Electrochim. Acta. 55, 7484–7488 (2010).
K. Fic, G. Lota, and E. Frackowiak, Effect of Surfactants on Capacitance Properties of Carbon Electrodes. Electrochim. Acta. 60, 206–212 (2012).
P.W. Ruch, D. Cericola, A. Foelske-Schmitz, R. Kötz, and A. Wokaun, Aging of Electrochemical Double Layer Capacitors with Acetonitrile-Based Electrolyte at Elevated Voltages. Electrochim. Acta. 55, 4412–4420 (2010).
E. Raymundo-Piñero, K. Kierzek, J. Machnikowski, and F. Béguin, Relationship Between the Nanoporous Texture of Activated Carbons and Their Capacitance Properties in Different Electrolytes. Carbon N. Y. 44, 2498–2507 (2006).
T. Abdallah, D. Lemordant, and B. Claude-Montigny, Are Room Temperature Ionic Liquids Able to Improve the Safety of Supercapacitors Organic Electrolytes Without Degrading the Performances? J. Power Sources. 201, 353–359 (2012).
T. Sato, G. Masuda, and K. Takagi, Electrochemical Properties of Novel Ionic Liquids for Electric Double Layer Capacitor Applications. Electrochim. Acta. 49, 3603–3611 (2004).
C.A. Angell, Y. Ansari, and Z. Zhao, Ionic Liquids: Past, Present and Future. Faraday Discuss. 154, 9–27 (2012).
M. Galiński, A. Lewandowski, and I. Stepniak, Ionic Liquids as Electrolytes. Electrochim. Acta. 51, 5567–5580 (2006).
A. Lewandowski and A. Świderska-Mocek, Ionic Liquids As Electrolytes for Li-ion Batteries—An Overview of Electrochemical Studies. J. Power Sources. 194, 601–609 (2009).
K. Perzyna, R. Borkowska, J. Syzdek, A. Zalewska, and W. Wieczorek, The Effect of Additive of Lewis Acid Type on Lithium-Gel Electrolyte Characteristics. Electrochim. Acta. 57, 58–65 (2011).
J. Syzdek, R. Borkowska, K. Perzyna, J.M. Tarascon, and W. Wieczorek, Novel Composite Polymeric Electrolytes with Surface-Modified Inorganic Fillers. J. Power Sources. 173, 712–720 (2007).
J. Luo, A.H. Jensen, N.R. Brooks, J. Sniekers, M. Knipper, D. Aili, Q. Li, B. Vanroy, M. Wübbenhorst, F. Yan, L. Van Meervelt, Z. Shao, J. Fang, Z.H. Luo, D.E. De Vos, K. Binnemans, and J. Fransaer, 1,2,4-Triazolium Perfluorobutanesulfonate as an Archetypal Pure Protic Organic Ionic Plastic Crystal Electrolyte for All-Solid-State Fuel Cells. Energy Environ. Sci. 8, 1276–1291 (2015).
J. Luo, O. Conrad, and I.F.J. Vankelecom, Imidazolium Methanesulfonate As A High Temperature Proton Conductor. J. Mater. Chem. A. 1, 2238–2247 (2013).
S. Wu, Y. Xue, Q. Yang, Q. Hu, T. Cui, Q. Su, F. Yin, Y. Wang, and H. Zhan, Conductive Carbon Spheres-Supported Nickel-Cobalt Selenide Nanoparticles as A High-Performance and Long-Life Electrode for Supercapacitors. Diam. Relat. Mater. 111, 108187 (2021).
J. Yu, J. Wu, H. Wang, A. Zhou, C. Huang, H. Bai, and L. Li, Metallic Fabrics As the Current Collector for High-Performance Graphene-Based Flexible Solid-State Supercapacitor. ACS Appl. Mater. Interfaces. 8, 4724–4729 (2016).
K.O. Oyedotun, T.M. Masikhwa, S. Lindberg, A. Matic, P. Johansson, and N. Manyala, Comparison of Ionic Liquid Electrolyte to Aqueous Electrolytes on Carbon Nanofibres Supercapacitor Electrode Derived from Oxygen-Functionalized Graphene. Chem. Eng. J. 375, 121906 (2019).
Q. Wang, J. Xu, X. Wang, B. Liu, X. Hou, G. Yu, P. Wang, D. Chen, and G. Shen, Core-Shell CuCo2O4@MnO2 Nanowires on Carbon Fabrics as High-Performance Materials for Flexible All-Solid-State, Electrochemical Capacitors. ChemElectroChem. 1, 559–564 (2014).
G.P. Pandey, T. Liu, C. Hancock, Y. Li, X.S. Sun, and J. Li, Thermostable Gel Polymer Electrolyte Based on Succinonitrile and Ionic Liquid for High-Performance Solid-State Supercapacitors. J. Power Sources. 328, 510–519 (2016).
L. Han, H. Huang, X. Fu, J. Li, Z. Yang, X. Liu, L. Pan, and M. Xu, A Flexible, High-Voltage and Safe Zwitterionic Natural Polymer Hydrogel Electrolyte for High-Energy-Density Zinc-Ion Hybrid Supercapacitor. Chem. Eng. J. 392, 123733 (2020).
A.M. Patil, N. Kitiphatpiboon, X. An, X. Hao, S. Li, X. Hao, A. Abudula, and G. Guan, Fabrication of a High-Energy Flexible all-Solid-State Supercapacitor Using Pseudocapacitive 2D-Ti3C2T x-MXene and Battery-Type Reduced Graphene Oxide/Nickel-Cobalt Bimetal Oxide Electrode Materials. ACS Appl. Mater. Interfaces 12, 52749–52762 (2020).
N.R. Chodankar, D.P. Dubal, A.C. Lokhande, and C.D. Lokhande, Ionically Conducting PVA–LiClO4 Gel Electrolyte for High Performance Flexible Solid-State Supercapacitors. J. Colloid Interface Sci. 460, 370–376 (2015).
Y. Lv, L. Li, Y. Zhou, M. Yu, J. Wang, J. Liu, J. Zhou, Z. Fan, and Z. Shao, A Cellulose-Based Hybrid 2D Material Aerogel for a Flexible All-Solid-State Supercapacitor with High Specific Capacitance. RSC Adv. 7, 43512–43520 (2017).
P. Du, X. Hu, C. Yi, H.C. Liu, P. Liu, H.-L. Zhang, X. Gong, P.C. Du, X. Hu, C. Yi, H.C. Liu, X. Gong, P. Liu, and H. Zhang, Self-Powered Electronics by Integration of Flexible Solid-State Graphene-Based Supercapacitors with High Performance Perovskite Hybrid Solar Cells. Adv. Funct. Mater. 25, 2420–2427 (2015).
P. Arora and Z. Zhang, Battery Separators. Chem. Rev. 104, 4419–4462 (2004).
S.S. Zhang, A review on the Separators of Liquid Electrolyte Li-ion Batteries. J. Power Sources. 164, 351–364 (2007).
K.D. Verma, Characteristics of separator materials for supercapacitors, Handbook Nanocomposite Supercapacitor Materials I. (Cham: Springer, 2020), pp. 315–326.
P. Zhu, D. Gastol, J. Marshall, R. Sommerville, V. Goodship, and E. Kendrick, A Review of Current Collectors for Lithium-Ion Batteries. J. Power Sources. 485, 229321 (2021). https://doi.org/10.1016/j.jpowsour.2020.229321.
A.S. Aricò, P. Bruce, B. Scrosati, J.-M. Tarascon, and W. van Schalkwijk, Nanostructured Materials for Advanced Energy Conversion and Storage Devices. Nat. Mater. 4, 366–377 (2005).
O. Barbieri, M. Hahn, A. Herzog, and R. Kötz, Capacitance Limits of High Surface Area Activated Carbons for Double Layer Capacitors. Carbon N. Y. 43, 1303–1310 (2005).
S. Rajagopal, R. Pulapparambil Vallikkattil, M. Mohamed Ibrahim, and D.G. Velev, Electrode Materials for Supercapacitors in Hybrid Electric Vehicles: Challenges and Current Progress. Condens. Matter 7, 6 (2022).
C. Largeot, C. Portet, J. Chmiola, P.L. Taberna, Y. Gogotsi, and P. Simon, Relation Between the Ion Size and Pore Size for an Electric Double-Layer Capacitor. J. Am. Chem. Soc. 130, 2730–2731 (2008).
H. Lee, M.S. Cho, I.H. Kim, J. Do Nam, and Y. Lee, RuOx/Polypyrrole Nanocomposite Electrode for Electrochemical Capacitors. Synth. Met. 160, 1055–1059 (2010).
D. Deng, Li-Ion Batteries: Basics, Progress, and Challenges. Energy Sci. Eng. 3, 385–418 (2015).
F. Barzegar, Supercapacitor or Battery, (n.d.), SA Energy Storage, ee publishers (2018). https://www.ee.co.za/wp-content/uploads/2018/10/Dr-Farshad-Barzegar-University-of-Pretoria-presentation.pdf
H. Pan, J. Li, and Y.P. Feng, Carbon Nanotubes for Supercapacitor. Nanoscale Res. Lett. 5, 654–668 (2010).
H. Zhang, G. Cao, and Y. Yang, Carbon Nanotube Arrays and Their Composites for Electrochemical Capacitors and Lithium-Ion Batteries. Energy Environ. Sci. 2, 932–943 (2009).
G. Wang, L. Zhang, and J. Zhang, A Review of Electrode Materials for Electrochemical Supercapacitors. Chem. Soc. Rev. 41, 797–828 (2012).
Y. Gogotsi, A. Nikitin, H. Ye, W. Zhou, J.E. Fischer, B. Yi, H.C. Foley, and M.W. Barsoum, Nanoporous Carbide-Derived Carbon with Tunable Pore Size. Nat. Mater. 2, 591–594 (2003).
V. Presser, M. Heon, and Y. Gogotsi, Carbide-Derived Carbons - from Porous Networks to Nanotubes and Graphene. Adv. Funct. Mater. 21, 810–833 (2011).
G.N. Yushin, E.N. Hoffman, A. Nikitin, H. Ye, M.W. Barsoum, and Y. Gogotsi, Synthesis of Nanoporous Carbide-Derived Carbon by Chlorination of Titanium Silicon Carbide. Carbon N. Y. 43, 2075–2082 (2005).
K.O. Oyedotun and N. Manyala, Graphene Foam–Based Electrochemical Capacitors. Curr. Opin. Electrochem. 21, 125–131 (2020).
C. Peng, J. Jin, and G.Z. Chen, A Comparative Study on Electrochemical co-Deposition and Capacitance of Composite Films of Conducting Polymers and Carbon Nanotubes. Electrochim. Acta. 53, 525–537 (2007).
C. Arbizzani, M. Mastragostino, and L. Meneghello, Polymer-Based Redox Supercapacitors: A Comparative Study. Electrochimica. Acta. 41, 21–26 (1996).
M. Kalaji, P.J. Murphy, and G.O. Williams, The Study of Conducting Polymers for Use as Redox Supercapacitors. Synth. Met. 102, 1360–1361 (1999).
W. Li, J. Chen, J. Zhao, J. Zhang, and J. Zhu, Application of Ultrasonic Irradiation in Preparing Conducting Polymer as Active Materials for Supercapacitor. Mater. Lett. 59, 800–803 (2005).
H. Li, J. Wang, Q. Chu, Z. Wang, F. Zhang, and S. Wang, Theoretical and Experimental Specific Capacitance of Polyaniline in Sulfuric Acid. J. Power Sources. 190, 578–586 (2009).
M. Deschamps, E. Gilbert, P. Azais, E. Raymundo-Piñero, M.R. Ammar, P. Simon, D. Massiot, and F. Béguin, Exploring Electrolyte Organization in Supercapacitor Electrodes with Solid-State NMR. Nat. Mater. 12, 351–358 (2013).
J.P. Zheng, The Limitations of Energy Density for Electrochemical Capacitors. J. Electrochem. Soc. 144, 2026 (1997).
T. Liu, W.G. Pell, and B.E. Conway, Self-Discharge and Potential Recovery Phenomena at Thermally and Electrochemically Prepared RuO2 Supercapacitor Electrodes. Electrochim. Acta. 42, 3541–3552 (1997).
Y.R. Ahn, M.Y. Song, S.M. Jo, C.R. Park, and D.Y. Kim, Electrochemical Capacitors Based on Electrodeposited Ruthenium Oxide on Nanofibre Substrates. Nanotechnology 17, 2865–2869 (2006).
M. Toupin, T. Brousse, and D. Bélanger, Charge Storage Mechanism of MnO2 Electrode Used in Aqueous Electrochemical Capacitor. Chem. Mater. 16, 3184–3190 (2004).
K.-C. Liu, Porous Nickel Oxide/Nickel Films for Electrochemical Capacitors. J. Electrochem. Soc. 143, 124 (1996).
Y. Gao, S. Chen, D. Cao, G. Wang, and J. Yin, Electrochemical Capacitance of Co3O4 Nanowire Arrays Supported on Nickel Foam. J. Power Sources. 195, 1757–1760 (2010).
N. Miura, S. Oonishi, and K.R. Prasad, Indium Tin Oxide/Carbon Composite Electrode Material for Electrochemical Supercapacitors. Electrochem. Solid-State Lett. 7, A247 (2004).
X. Zhou, H. Chen, D. Shu, C. He, and J. Nan, Study on the Electrochemical Behavior of Vanadium Nitride as a Promising Supercapacitor Material. J. Phys. Chem. Solids 70, 495–500 (2009).
J.P. Cheng, J. Zhang, and F. Liu, Recent Development of Metal Hydroxides as Electrode Material of Electrochemical Capacitors. RSC Adv. 4, 38893–38917 (2014).
W. Zhang, F. Liu, Q. Li, Q. Shou, J. Cheng, L. Zhang, B.J. Nelson, and X. Zhang, Transition Metal Oxide and Graphene Nanocomposites for High-Performance Electrochemical Capacitors. Phys. Chem. Chem. Phys. 14, 16331 (2012).
H. Chen, L. Hu, Y. Yan, R. Che, M. Chen, and L. Wu, One-Step Fabrication of Ultrathin Porous Nickel Hydroxide-Manganese Dioxide Hybrid Nanosheets for Supercapacitor Electrodes with Excellent Capacitive Performance. Adv. Energy Mater. 3, 1636–1646 (2013).
L. Feng, Y. Zhu, H. Ding, and C. Ni, Recent Progress in Nickel Based Materials for High Performance Pseudocapacitor Electrodes. J. Power Sources. 267, 430–444 (2014).
A.A. Grupioni, E. Arashiro, and T.A. Lassali, Voltammetric Characterization of an Iridium Oxide-Based System: The Pseudocapacitive Nature of the Ir0.3Mn0.7O2 Electrode. Electrochim. Acta. 48, 407–418 (2002).
A. Borenstein, O. Hanna, R. Attias, S. Luski, T. Brousse, and D. Aurbach, Carbon-Based Composite Materials for Supercapacitor Electrodes: A Review. J. Mater. Chem. A. 5, 12653–12672 (2017).
R. Sahoo, T.H. Lee, D.T. Pham, T.H.T. Luu, and Y.H. Lee, Fast-Charging High-Energy Battery-Supercapacitor Hybrid: Anodic Reduced Graphene Oxide-VANADIUM(IV) Oxide Sheet-on-Sheet Heterostructure. ACS Nano. 13, 10776–10786 (2019).
Y. Zhou, K. Maleski, B. Anasori, J.O. Thostenson, Y. Pang, Y. Feng, K. Zeng, C.B. Parker, S. Zauscher, Y. Gogotsi, J.T. Glass, and C. Cao, Ti3C2Tx MXene-Reduced Graphene Oxide Composite Electrodes for Stretchable Supercapacitors. ACS Nano. 14, 3576–3586 (2020).
J. Li, X. Cheng, A. Shashurin, M. Keidar, J. Li, X. Cheng, A. Shashurin, and M. Keidar, Review of Electrochemical Capacitors Based on Carbon Nanotubes and Graphene. Graphene 1, 1–13 (2012).
M. Toupin, T. Brousse, and D. Bélanger, Influence of Microstucture on the Charge Storage Properties of Chemically Synthesized Manganese Dioxide. Chem. Mater. 14, 3946–3952 (2002).
T. Cottineau, M. Toupin, T. Delahaye, T. Brousse, and D. Bélanger, Nanostructured Transition Metal Oxides for Aqueous Hybrid Electrochemical Supercapacitors. Appl. Phys. A Mater. Sci. Process. 82, 599–606 (2006).
T. Brousse, D. Belanger, and J.W. Long, To Be or Not To Be Pseudocapacitive? J. Electrochem. Soc. 162, A5185–A5189 (2015).
W. Deng, X. Ji, Q. Chen, and C.E. Banks, Electrochemical Capacitors Utilising Transition Metal Oxides: an Update of Recent Developments. RSC Adv. 1, 1171–1178 (2011).
M.Y. Ho, P.S. Khiew, D. Isa, T.K. Tan, W.S. Chiu, and C.H. Chia, A Review of Metal Oxide Composite Electrode Materials for Electrochemical Capacitors. NANO 9, 1–25 (2014).
C.Q. Yi, J.P. Zou, H.Z. Yang, and L. Xian, Recent Advances in Pseudocapacitor Electrode Materials: Transition Metal Oxides and Nitrides. Trans. Nonferrous Metals Soc. Chin. 28, 1980–2001 (2018).
B. Anasori, M.R. Lukatskaya, and Y. Gogotsi, 2D Metal Carbides and Nitrides (MXenes) for Energy Storage. Nat. Rev. Mater. 2, 1–17 (2017).
A. Djire, A. Bos, J. Liu, H. Zhang, E.M. Miller, and N.R. Neale, Pseudocapacitive Storage in Nanolayered Ti2NTx MXene Using Mg-Ion Electrolyte. ACS Appl. Nano Mater. 2, 2785–2795 (2019).
B. Anasori, M.R. Lukatskaya, and Y. Gogotsi, 2D Metal Carbides and Nitrides (MXenes) for Energy Storage. Nat. Rev. Mater. 2, 16098 (2017).
B. Das, M. Behm, G. Lindbergh, M.V. Reddy, and B.V.R. Chowdari, High Performance Metal Nitrides, MN (M = Cr, Co) Nanoparticles for Non-Aqueous Hybrid Supercapacitors. Adv. Powder Technol. 26, 783–788 (2015).
C. Zhu, P. Yang, D. Chao, X. Wang, X. Zhang, S. Chen, and H.J. Fan, All Metal Nitrides Solid-State Asymmetric Supercapacitors. Adv. Mater. 27, 4566–4571 (2015).
O. Sahnoun, H. Bouhani-Benziane, M. Sahnoun, and M. Driz, Magnetic and Thermoelectric Properties of Ordered Double Perovskite Ba2FeMoO6. J. Alloys Compd. 714, 704–708 (2017).
I. Sfifir, A. Ezaami, W. Cheikhrouhou-Koubaa, and A. Cheikhrouhou, Critical Properties in Dy-doped La0.7−xDyxSr0.3MnO.3 (x=0.00, 0.03) Manganites. Ceram. Int. 43, 8784–8791 (2017).
P. Forouzandeh and S.C. Pillai, Two-Dimensional (2D) Electrode Materials for Supercapacitors. Mater. Today Proc. 41, 498–505 (2020).
G. Kim, S. Wang, A.J. Jacobson, L. Reimus, P. Brodersen, and C.A. Mims, Rapid Oxygen ion Diffusion and Surface Exchange Kinetics in PrBaCo2O5+x with a Perovskite Related Structure and Ordered A Cations. J. Mater. Chem. 17, 2500–2505 (2007).
G. George, S.L. Jackson, C.Q. Luo, D. Fang, D. Luo, D. Hu, J. Wen, and Z. Luo, Effect of Doping on the Performance of High-Crystalline SrMnO3 Perovskite Nanofibers as a Supercapacitor Electrode. Ceram. Int. 44, 21982–21992 (2018).
P.P. Ma, B. Zhu, N. Lei, Y.K. Liu, B. Yu, Q.L. Lu, J.M. Dai, S.H. Li, and G.H. Jiang, Effect of Sr Substitution on Structure and Electrochemical Properties of Perovskite-Type LaMn0.9Ni0.1O3 Nanofibers. Mater. Lett. 252, 23–26 (2019).
A. Navrotsky, Energetics and Crystal Chemical Systematic Among Ilmenite, Lithium Niobate, and Perovskite Structures. Chem. Mater. 10, 2787–2793 (1998).
X. Li and H. Zhu, Two-Dimensional MoS2: Properties, Preparation, and Applications. J. Mater. 1, 33–44 (2015).
H. Zhang, Ultrathin Two-Dimensional Nanomaterials. ACS Nano Lecturesh. Award. 9, 9451–9469 (2015).
S. Das, J.A. Robinson, M. Dubey, H. Terrones, and M. Terrones, Beyond Graphene: Progress in Novel Two-Dimensional Materials and Van Der Waals SOLIDS. Annu. Rev. Mater. Res. (2015). https://doi.org/10.1146/Annurev-Matsci-070214-021034.
X. Huang, Z. Zeng, and H. Zhang, Metal Dichalcogenide Nanosheets: Preparation, Properties and Applications. Chem. Soc. Rev. 42, 1934–1946 (2013).
J. Feng, X. Sun, C. Wu, L. Peng, C. Lin, S. Hu, J. Yang, and Y. Xie, Metallic Few-Layered VS2 Ultrathin Nanosheets: High Two-Dimensional Conductivity for in-Plane Supercapacitors. J. Am. Chem. Soc. 133, 17832–17838 (2011).
A.P. Cote, A.I. Benin, N.W. Ockwig, M. O’Keeffe, A.J. Matzger, and O.M. Yaghi, Porous Crystalline Covalent Organic Frameworks. Science 310, 1166–1170 (2005).
H. Ding, A. Mal, and C. Wang, Tailored Covalent Organic Frameworks by Post-Synthetic Modification. Mater. Chem. Front. 4, 113–127 (2019).
C. Krishnaraj, H. Sekhar Jena, L. Bourda, A. Laemont, P. Pachfule, J. Roeser, and P. Van Der Voort, Strongly Reducing (diarylamino) Benzene-Based Covalent Organic Framework for Metal-Free Visible light Photocatalytic H2O2 Generation. J. Am. Chem. Soc. 142, 20107–20116 (2020).
S. Chandra, D. Roy Chowdhury, M. Addicoat, T. Heine, A. Paul, and R. Banerjee, Molecular Level Control of the Capacitance of Two-Dimensional Covalent Organic Frameworks: Role of Hydrogen Bonding in Energy Storage Materials. Chem. Mater. 29, 2074–2080 (2017).
File:2D COF conductivity.jpg - Wikimedia Commons, (n.d.). https://commons.wikimedia.org/wiki/File:2D_COF_conductivity.jpg#filelinks (accessed April 25, 2022).
File:A chemical structure of the DAAQ-TFP covalent organic framework.png - Wikimedia Commons,(n.d.).https://commons.wikimedia.org/wiki/File:A_chemical_structure_of_the_DAAQ-TFP_covalent_organic_framework.png (accessed April 25, 2022).
W. Yang, Z. Gao, J. Wang, J. Ma, M. Zhang, and L. Liu, Solvothermal One-Step Synthesis of Ni − Al Layered Double Hydroxide / Carbon Nanotube / Reduced Graphene Oxide Sheet Ternary Nanocomposite with Ultrahigh Capacitance for Supercapacitors. ACS Appl. Mater. Interfaces 5, 5443–5454 (2013).
Metal-organic framework structure, molecular model - Stock Image - C049/3167 - Science Photo Library, (n.d.). https://www.sciencephoto.com/media/1120025/view (accessed March 30, 2022).
MOF Metal Organic Framework - definition, fabrication and use, (n.d.). https://www.nanowerk.com/mof-metal-organic-framework.php (accessed April 26, 2022).
B.Y. Xia, Y. Yan, N. Li, H.B. Wu, X.W.D. Lou, and X. Wang, A Metal–Organic Framework-Derived Bifunctional Oxygen Electrocatalyst. Nat. Energy 1, 1–8 (2016).
R.R. Salunkhe, J. Tang, Y. Kamachi, T. Nakato, J.H. Kim, and Y. Yamauchi, Asymmetric Supercapacitors Using 3D Nanoporous carbon and Cobalt Oxide Electrodes Synthesized from a Single Metal-Organic Framework. ACS Nano 9, 6288–6296 (2015).
G. Huang, F. Zhang, X. Du, Y. Qin, D. Yin, and L. Wang, Metal Organic Frameworks Route to in Situ Insertion of Multiwalled Carbon Nanotubes in Co3o4polyhedra as Anode Materials for Lithium-Ion Batteries. ACS Nano 9, 1592–1599 (2015).
L. Wang, Y. Han, X. Feng, J. Zhou, P. Qi, and B. Wang, Metal–Organic Frameworks for Energy Storage: Batteries and Supercapacitors. Coord. Chem. Rev. 307, 361–381 (2016).
M. Naguib, J. Halim, J. Lu, K.M. Cook, L. Hultman, Y. Gogotsi, and M.W. Barsoum, New Two-Dimensional Niobium and Vanadium Carbides as Promising Materials for Li-Ion Batteries. J. Am. Chem. Soc. 135, 15966–15969 (2013).
M. Ghidiu, M. Naguib, C. Shi, O. Mashtalir, L.M. Pan, B. Zhang, J. Yang, Y. Gogotsi, S.J.L. Billinge, and M.W. Barsoum, Synthesis and Characterization of Two-Dimensional Nb4C3 (MXene). Chem. Commun. 50, 9517–9520 (2014).
J.C. Gui, L. Han, and W.Y. Cao, Lamellar MXene: A Novel 2D Nanomaterial for Electrochemical Sensors. J. Appl. Electrochem. 51, 1509–1522 (2021).
B. Anasori, Y. Xie, M. Beidaghi, J. Lu, B.C. Hosler, L. Hultman, P.R.C. Kent, Y. Gogotsi, and M.W. Barsoum, Two-Dimensional, Ordered, Double Transition Metals Carbides (MXenes). ACS Nano 9, 9507–9516 (2015).
M. Naguib, V.N. Mochalin, M.W. Barsoum, and Y. Gogotsi, 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 26, 992–1005 (2014).
J. Chen, W. Yan, E.J. Townsend, J. Feng, L. Pan, V. Del Angel Hernandez, and C.F. Faul, Tunable Surface Area, Porosity, and Function in Conjugated Microporous Polymers. Angewandte Chemie Int. Ed. 58, 11715–11719 (2019).
J. Chen, T. Qiu, W. Yan, and C.F.J. Faul, Exploiting Hansen Solubility Parameters to Tune Porosity and Function in Conjugated Microporous Polymers. J. Mater. Chem. A 8, 22657–22665 (2020).
K. Amin, N. Ashraf, L. Mao, C.F.J. Faul, and Z. Wei, Conjugated Microporous Polymers for Energy Storage: Recent Progress and Challenges. Nano Energy 85, 105958 (2021).
F. Xu, X. Chen, Z. Tang, D. Wu, R. Fu, and D. Jiang, Redox-Active Conjugated Microporous Polymers: A New Organic Platform for Highly Efficient Energy Storage. Chem. Commun. 50, 4788–4790 (2014).
J.S.M. Lee and A.I. Cooper, Advances in Conjugated Microporous Polymers. Chem. Rev. 120, 2171–2214 (2020).
A.I. Cooper, Conjugated Microporous Polymers. Adv. Mater. 21, 1291–1295 (2009).
K.O. Oyedotun, F. Barzegar, A.A. Mirghni, A.A. Khaleed, T.M. Masikhwa, and N. Manyala, Examination of High-Porosity Activated Carbon Obtained from Dehydration of White Sugar for Electrochemical Capacitor Applications. ACS Sustain. Chem. Eng. 7, 537–546 (2019).
K.O. Oyedotun, A.A. Mirghni, O. Fasakin, D.J. Tarimo, V.N. Kitenge, and N. Manyala, High-Energy Asymmetric Supercapacitor Based on the Nickel Cobalt Oxide (NiCo2O4) Nanostructure Material and Activated Carbon Derived from Cocoa Pods. Energy Fuels 35, 20309–20319 (2021).
R.B. Choudhary, S. Ansari, and B. Purty, Robust Electrochemical Performance of Polypyrrole (PPy) and Polyindole (PIn) Based Hybrid Electrode Materials for Supercapacitor Application: A Review. J. Energy Storage. 29, 101302 (2020).
N.M. Soudagar, V.K. Pandit, R.B. Pujari, K.B. Chorghade, C.D. Lokhande, and S.S. Joshi, Chemically Synthesized Polyaniline Supercapacitor. Int. J. Eng. Res. Technol. 10, 587–594 (2017).
M. Rajesh, C.J. Raj, R. Manikandan, B.C. Kim, S.Y. Park, and K.H. Yu, A High Performance PEDOT/PEDOT Symmetric Supercapacitor by Facile In-Situ Hydrothermal Polymerization of PEDOT Nanostructures on Flexible Carbon Fibre Cloth Electrodes. Mater. Today Energy 6, 96–104 (2017).
C. Zhao, X. Wang, S. Wang, H. Wang, Y. Yang, and W. Zheng, Pseudocapacitive Properties of Cobalt Hydroxide Electrodeposited on Ni-Foam-Supported Carbon Nanomaterial. Mater. Res. Bull. 48, 3189–3195 (2013).
H.B. Li, M.H. Yu, F.X. Wang, P. Liu, Y. Liang, J. Xiao, C.X. Wang, Y.X. Tong, and G.W. Yang, Amorphous Nickel Hydroxide Nanospheres with Ultrahigh Capacitance and Energy Density as Electrochemical Pseudocapacitor Materials. Nat. Commun. 4, 1894 (2013).
C. Han, H. Si, S. Sang, K. Liu, H. Liu, and Q. Wu, Carbon Dots Doped with Ni(OH)2as Thin-Film Electrodes for Supercapacitors. ACS Appl. Nano Mater. 3, 12106–12114 (2020).
Y. Song, X. Cai, X. Xu, and X.X. Liu, Integration of Nickel–Cobalt Double Hydroxide Nanosheets and Polypyrrole Films with Functionalized Partially Exfoliated Graphite for Asymmetric Supercapacitors with Improved Rate Capability. J. Mater. Chem. A. 3, 14712–14720 (2015).
S. Ramesh, K. Karuppasamy, H.M. Yadav, J.J. Lee, H.S. Kim, H.S. Kim, and J.H. Kim, Ni (OH) 2-Decorated Nitrogen Doped MWCNT Nanosheets as An Efficient Electrode for High Performance Supercapacitors. Sci. Rep. 9, 1–10 (2019).
X. He, W. Yang, X. Mao, L. Xu, Y. Zhou, Y. Chen, Y. Zhao, Y. Yang, and J. Xu, All-Solid State Symmetric Supercapacitors Based on Compressible and Flexible Free-Standing 3D Carbon Nanotubes (CNTs)/Poly(3,4-ethylenedioxythiophene) (PEDOT) Sponge Electrodes. J. Power Sources. 376, 138–146 (2018).
G. Wu, P. Tan, D. Wang, Z. Li, L. Peng, Y. Hu, C. Wang, W. Zhu, S. Chen, and W. Chen, High-Performance Supercapacitors Based on Electrochemical-Induced Vertical-Aligned Carbon Nanotubes and Polyaniline Nanocomposite Electrodes. Sci. Rep. 7, 1–8 (2017).
R. Xue, Y.P. Zheng, D.Q. Qian, D.Y. Xu, Y.S. Liu, S.L. Huang, and G.Y. Yang, A 2-D Microporous Covalent Organic Framework for High-Performance Supercapacitor Electrode. Mater. Lett. 308, 131229 (2022).
R. Iqbal, A. Badshah, Y.J. Ma, and L.J. Zhi, An Electrochemically Stable 2D Covalent Organic Framework for High-Performance Organic Supercapacitors. Chin. J. Polym. Sci. 38, 558–564 (2020).
N. An, Z. Guo, J. Xin, Y. He, K. Xie, D. Sun, X. Dong, and Z. Hu, Hierarchical Porous Covalent Organic Framework/Graphene Aerogel Electrode for High-Performance Supercapacitors. J. Mater. Chem. A 9, 16824–16833 (2021).
D.J. Li, S. Lei, Y.Y. Wang, S. Chen, Y. Kang, Z.G. Gu, and J. Zhang, Helical Carbon Tubes Derived from Epitaxial Cu-MOF Coating on Textile for Enhanced Supercapacitor Performance. Dalt. Trans. 47, 5558–5563 (2018).
C. Qu, Y. Jiao, B. Zhao, D. Chen, R. Zou, K.S. Walton, and M. Liu, Nickel-Based Pillared MOFs for High-Performance Supercapacitors: Design, Synthesis and Stability Study. Nano Energy 26, 66–73 (2016).
S. Venkateshalu, J. Cherusseri, M. Karnan, K.S. Kumar, P. Kollu, M. Sathish, J. Thomas, S.K. Jeong, and A.N. Grace, New Method for the Synthesis of 2D Vanadium Nitride (MXene) and its Application as a Supercapacitor Electrode. ACS Omega 5, 17983–17992 (2020).
K.O. Oyedotun, D.Y. Momodu, M. Naguib, A.A. Mirghni, T.M. Masikhwa, A.A. Khaleed, and N. Manyala, Electrochemical Performance of Two-Dimensional Ti3C2-Mn3O4 Nanocomposites and Carbonized Iron Cations for Hybrid Supercapacitor Electrodes. Electrochimica. Acta. 301, 487–499 (2019).
Q. Shan, X. Mu, M. Alhabeb, C.E. Shuck, D. Pang, X. Zhao, and Y. Dall’Agnese, Two-Dimensional Vanadium Carbide (V2C) MXene as Electrode for Supercapacitors with Aqueous Electrolytes. Electrochem. Commun. 96, 103–107 (2018).
W. Lyu, W. Zhang, H. Liu, Y. Liu, H. Zuo, C. Yan, C.F.J. Faul, A. Thomas, M. Zhu, and Y. Liao, Conjugated Microporous Polymer Network Grafted Carbon Nanotube Fibers with Tunable Redox Activity for Efficient Flexible Wearable Energy Storage. Chem. Mater. 32, 8276–8285 (2020).
Y. Kou, Y. Xu, Z. Guo, and D. Jiang, Supercapacitive Energy Storage and Electric Power Supply Using an aza-Fused π-Conjugated Microporous Framework. Angew. Chemie. 123, 8912–8916 (2011).
Y. Liao, H. Wang, M. Zhu, A. Thomas, Y. Liao, H. Wang, M. Zhu, and A. Thomas, Efficient Supercapacitor Energy Storage Using Conjugated Microporous Polymer Networks Synthesized from Buchwald-Hartwig Coupling. Adv. Mater. 30, 1705710 (2018).
J. Fischer, K. Thümmler, S. Fischer, I.G. Gonzalez Martinez, S. Oswald, and D. Mikhailova, Activated Carbon Derived from Cellulose and Cellulose Acetate Microspheres as Electrode Materials for Symmetric Supercapacitors in Aqueous Electrolytes. Energy Fuels 35, 12653–12665 (2021).
M.N. Rantho, M.J. Madito, and N. Manyala, Symmetric Supercapacitor with Supercapattery Behavior Based on Carbonized Iron Cations Adsorbed Onto Polyaniline. Electrochim. Acta. 262, 82–96 (2018).
A. Bello, F. Barzegar, M.J. Madito, D.Y. Momodu, A.A. Khaleed, T.M. Masikhwa, J.K. Dangbegnon, and N. Manyala, Electrochemical Performance of Polypyrrole Derived Porous Activated Carbon-Based Symmetric Supercapacitors in Various Electrolytes. RSC Adv. 6, 68141–68149 (2016).
Y. Zhou, P. Jin, Y. Zhou, and Y. Zhu, High-Performance Symmetric Supercapacitors Based on Carbon Nanotube/Graphite Nanofiber Nanocomposites. Sci. Rep. 8, 1–8 (2018).
Y. Ma, D. Chen, Z. Fang, Y. Zheng, W. Li, S. Xu, X. Lu, G. Shao, Q. Liu, and W. Yang, High Energy Density and Extremely Stable Supercapacitors Based on Carbon Aerogels with 100% Capacitance Retention up to 65,000 Cycles. Proc. Natl. Acad. Sci. U. S. A. 118, 1–8 (2021).
M. Ibrahim, H.N. Abdelhamid, A.M. Abuelftooh, S.G. Mohamed, Z. Wen, and X. Sun, Covalent Organic Frameworks (COFs)-Derived Nitrogen-Doped Carbon/Reduced Graphene Oxide Nanocomposite as Electrodes Materials for Supercapacitors. J. Energy Storage 55, 105375 (2022).
M.G. Mohamed, S.V. Chaganti, S.U. Sharma, M.M. Samy, M. Ejaz, J.T. Lee, and S.W. Kuo, Constructing Conjugated Microporous Polymers Containing the Pyrene-4, 5, 9, 10-Tetraone Unit for Energy Storage. ACS Appl. Energy Mater. 5, 10130–10140 (2022).
M.G. Mohamed, T.H. Mansoure, M.M. Samy, Y. Takashi, A.A.K. Mohammed, T. Ahamad, S.M. Alshehri, J. Kim, B.M. Matsagar, K.C.W. Wu, and S.W. Kuo, Ultrastable Conjugated Microporous Polymers Containing Benzobisthiadiazole and Pyrene Building Blocks for Energy Storage Applications. Molecules 27, 2025 (2022).
A. Bello, F. Barzegar, M.J. Madito, D.Y. Momodu, A.A. Khaleed, T.M. Masikhwa, J.K. Dangbegnon, and N. Manyala, Stability Studies of Polypyrole- Derived Carbon Based Symmetric Supercapacitor Via Potentiostatic Floating Test. Electrochim. Acta. 213, 107–114 (2016).
A. Bello, K. Makgopa, M. Fabiane, D. Dodoo-Ahrin, K.I. Ozoemena, and N. Manyala, Chemical Adsorption of NiO Nanostructures on Nickel Foam-Graphene for Supercapacitor Applications. J. Mater. Sci. 48, 6707–6712 (2013).
M. Inagaki, H. Konno, and O. Tanaike, Carbon Materials for Electrochemical Capacitors. J. Power Sources. 195, 7880–7903 (2010).
D. Weingarth, A. Foelske-Schmitz, A. Wokaun, and R. Kötz, PTFE Bound Activated Carbon - A Quasi-Reference Electrode for Ionic Liquids. Electrochem. Commun. 18, 116–118 (2012).
D. Weingarth, A. Foelske-Schmitz, A. Wokaun, and R. Kotz, PTFE Bound Activated Carbon - A Quasi Reference Electrode for Ionic Liquids and its Application. ECS Trans. 50, 111–117 (2013).
D.Y. Momodu, Investigation of metal hydroxides-graphene composites as electrode materials for supercapacitor applications., PhD diss. University of Pretoria (2015). http://hdl.handle.net/2263/50281
J.R. Miller, A.F. Burke, Electric vehicle capacitor test procedures manual, Idaho Natl. Eng. Lab. (1994).
M.D. Stoller and R.S. Ruoff, Best Practice Methods for Determining an Electrode Material’s Performance for Ultracapacitors. Energy Environ. Sci. 3, 1294–1301 (2010).
S. Shivakumara, B. Kishore, T.R. Penki, and N. Munichandraiah, Symmetric Supercapacitor Based on Partially Exfoliated and Reduced Graphite Oxide in Neutral Aqueous Electrolyte. Solid State Commun. 199, 26–32 (2014).
W. Zhang, C. Ma, J. Fang, J. Cheng, X. Zhang, S. Dong, and L. Zhang, Asymmetric Electrochemical Capacitors with High Energy and Power Density Based on Graphene/CoAl-LDH and Activated Carbon Electrodes. RSC Adv. 3, 2483 (2013).
K.O. Oyedotun, M.J. Madito, A. Bello, D.Y. Momodu, A.A. Mirghni, and N. Manyala, Investigation of Graphene Oxide Nanogel and Carbon Nanorods as Electrode for Electrochemical Supercapacitor. Electrochim. Acta. 245, 268–278 (2017).
P.L. Taberna, P. Simon, and J.F. Fauvarque, Electrochemical Characteristics and Impedance Spectroscopy Studies of Carbon-Carbon Supercapacitors. J. Electrochem. Soc. 150, A292 (2003).
B. Akinwolemiwa, C. Peng, and G.Z. Chen, Redox Electrolytes in Supercapacitors. J. Electrochem. Soc. 162, A5054–A5059 (2015).
C. Chukwuka, K.A. Folly, (2012). Batteries and super-capacitors. In IEEE Power and Energy Society Conference and Exposition in Africa: Intelligent Grid Integration of Renewable Energy Resources (PowerAfrica)
A. Lasia, Electrochemical impedance spectroscopy and its applications, Modern Aspects of Electrochemistry. (Boston: Springer, 2002), pp. 143–248.
S. Trasatti, Relative and Absolute Electrochemical Quantities. Components of the Potential Difference Across the Electrode/Solution Interface. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases. 70, 1752 (1974).
D.G. Grahame, The Electrical Double Layer and the Theory of Electrocapillarity. Chem. Rev. 41, 441–501 (1947).
W. Sun, R. Zheng, and X. Chen, Symmetric Redox Supercapacitor Based on Micro-Fabrication with Three-Dimensional Polypyrrole Electrodes. J. Power Sources. 195, 7120–7125 (2010).
J. Kowal, E. Avaroglu, F. Chamekh, A. Šenfelds, T. Thien, D. Wijaya, and D.U. Sauer, Detailed Analysis of the Self-Discharge of Supercapacitors. J. Power Sources. 196, 573–579 (2011).
F. Barzegar, Synthesis and characterization of activated carbon materials for supercapacitor applications, PhD diss. University of Pretoria (2016). http://hdl.handle.net/2263/53524
B.E. Conway, Similarities and differences between supercapacitors and batteries for storing electrical energy. In: Electrochemical Supercapacitors. (1999) 11–31.
A.G. Olabi, Q. Abbas, A. Al Makky, and M.A. Abdelkareem, Supercapacitors as Next Generation Energy Storage Devices: Properties and Applications. Energy 248, 123617 (2022).
J. Libich, J. Máca, J. Vondrák, O. Čech, and M. Sedlaříková, Supercapacitors: Properties and Applications. J. Energy Storage. 17, 224–227 (2018).
F. Bu, W. Zhou, Y. Xu, Y. Du, C. Guan, and W. Huang, Recent Developments of Advanced Micro-Supercapacitors: Design, Fabrication and Applications. Npj Flex. Electron. 4, 1–16 (2020).
T. Mesbahi, P. Bartholomeus, N. Rizoug, R. Sadoun, F. Khenfri, and P. Le Moigne, Advanced Model of Hybrid Energy Storage System Integrating Lithium-Ion Battery and Supercapacitor for Electric Vehicle Applications. IEEE Trans. Ind. Electron. 68, 3962–3972 (2021).
A.H.M. Aman, N. Shaari, and R. Ibrahim, Internet of Things Energy System: Smart Applications, Technology Advancement, and Open Issues. Int. J. Energy Res. 45, 8389–8419 (2021).
S. Ghosh, S.S. Withanage, B. Chamlagain, S.I. Khondaker, S. Harish, and B.B. Saha, Low Pressure Sulfurization and Characterization of Multilayer MoS2 for Potential Applications in Supercapacitors. Energy 203, 117918 (2020).
C.Y. Hsieh, P. Pei, Q. Bai, A. Su, F.B. Weng, and C.Y. Lee, Results of a 200 HOURS Lifetime Test of a 7 kW Hybrid-Power Fuel Cell System on Electric Forklifts. Energy 214, 118941 (2021).
A. Al-Zubaidi, X. Ji, and J. Yu, Thermal Charging of Supercapacitors: A Perspective, Sustain. Energy Fuels 1, 1457–1474 (2017).
K.V.G. Raghavendra, R. Vinoth, K. Zeb, C.V.M. Gopi, S. Sambasivam, M.R. Kummara, and H.J. Kim, An Intuitive Review of Supercapacitors with Recent Progress and Novel Device Applications. J. Energy Storage 31, 101652 (2020).
L. Wang, L. Wen, Y. Tong, S. Wang, X. Hou, X. An, S.X. Dou, and J. Liang, Photo-Rechargeable Batteries and Supercapacitors: Critical Roles of Carbon-Based Functional Materials. Carbon Energy 3, 225–252 (2021).
J.O. Ighalo, J.F. Amaku, C. Olisah, A.O. Adeola, K.O. Iwuozor, K.G. Akpomie, J. Conradie, K.A. Adegoke, and K.O. Oyedotun, Utilisation of Adsorption as a Resource Recovery Technique for Lithium in Geothermal Water. J. Mol. Liquids. 365, 120107 (2022).
A.O. Adeola, K.O. Iwuozor, K.G. Akpomie, K.A. Adegoke, K.O. Oyedotun, J.O. Ighalo, J.F. Amaku, C. Olisah, and J. Conradie, Advances in the Management of Radioactive Wastes and Radionuclide Contamination in Environmental Compartments: A Review. Environ. Geochem. Health. (2022). https://doi.org/10.1007/s10653-022-01378-7.
Acknowledgments
The authors are grateful to all publishers of articles from which some of the figures have been adapted and/or reproduced.
Funding
The research received no outside funding.
Author information
Authors and Affiliations
Contributions
OKO: Conceptualization, Methodology, Writing—original draft; Writing—review & editing; Supervision; Validation; Project administration (https://orcid.org/0000-0001-5081-6372) IJO: Writing—review & editing; Validation (https://orcid.org/0000-0002-8709-100X) AJF: Writing—review & editing; Validation (https://orcid.org/0000-0003-4894-0512) OC: Writing—review & editing; Validation (https://orcid.org/0000-0002-7714-3056) AAO: Writing—review & editing; Validation (https://orcid.org/0000-0002-7011-2396) IKO: Writing—review & editing; Validation (https://orcid.org/0000-0002-1161-2147) AKG: Writing—review & editing; Validation (https://orcid.org/0000-0003-2986-4294) CJ: Writing—review & editing; Validation (https://orcid.org/0000-0002-8120-6830) AKA: Writing—review & editing; Validation (https://orcid.org/0000-0002-7502-0132).
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest declared by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oyedotun, K.O., Ighalo, J.O., Amaku, J.F. et al. Advances in Supercapacitor Development: Materials, Processes, and Applications. J. Electron. Mater. 52, 96–129 (2023). https://doi.org/10.1007/s11664-022-09987-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-022-09987-9