Abstract
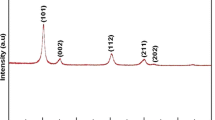
A mixture of anhydrous sodium sulfate, hydrated nickel phosphate, and sodium phosphate has been synthesized and various techniques used to characterize it. Differential scanning calorimetry was used to investigate the thermal properties in both O2 and N2 atmosphere at rate of 10 K min−1. The specific heat capacity was calculated from 298 K to 573 K and vice versa in two thermal cycles in both atmospheres, revealing values of 18,931.64 J kg−1 K−1 in O2 atmosphere and 15,568.39 J kg−1 K−1 in N2 atmosphere in the second thermal cycle, being exothermic in nature in both cases. This exothermic behavior of the mixture indicates its potential use as a heat-dissipating material. The crystallite size of this inorganic heat-dissipating mixture was found to be ~ 22.9 nm.
Similar content being viewed by others
References
C. Serre and G. Ferey, Inorg. Chem. 40, 5350 (2001).
S.C. Lin, S.Y. Chen, S.-Y. Cheng, and J.C. Lin, Solid State Sci. 7, 896 (2005).
A. Aaddane, M. Kacimi, and M. Ziyad, Catal. Lett. 73, 47 (2001).
S. Meseguer, M.A. Tena, C. Gargori, J.A. Nadenes, M. Llusar, and G. Monros, Ceram. Int. 33, 843 (2007).
R.D.A. Gaasbeek, H.G. Toonen, R.J. van Heerwaarden, and P. Buma, Biomaterials 26, 6713 (2005).
M.A. Deyab, K. Eddahaoui, R. Essehli, S. Benmokhtar, T. Rhadfi, A. De Riccardis, and G. Mele, J. Mol. Liq. 216, 699 (2016).
J. Zhao, S. Wang, Z. Run, G. Zhang, W. Du, and H. Pang, Part. Part. Syst. Charact. 32, 880 (2015).
D. Jian, Q. Gao, D. Gao, M. Ruan, and W. Shi, Phys. Lett. A 357, 136 (2006).
N. Guillou, Q. Gao, P.M. Forster, J.S. Chang, S.E. Park, G. Ferey, and A.K. Cheetham, Angew. Chem. Int. Ed. 40, 2831 (2001).
A.K. Cheetham, G. Ferey, and T. Loiseau, Angew. Chem. Int. Ed. 38, 3268 (1999).
N. Guillou, Q. Gao, M. Nogues, A.K. Cheetham, and G. Ferey, Solid State Sci. 4, 1179 (2002).
D. Gao and Q. Gao, Micropor. Mesopor. Mater. 85, 365 (2005).
F.S. Omar, A. Numan, N. Duraisamy, S. Bashir, K. Ramesh, and S. Ramesh, RSC Adv. 6, 76298 (2016).
T. Swain, J. Therm. Anal. Calorim. 127, 2191 (2017).
T. Swain and G.S. Brahma, J. Inorg. Organomet. Polym. 27, 131 (2017).
T. Swain, J. Ind. Chem. Soc. 91, 277 (2014).
T. Swain, J. Therm. Anal. Calorim. 110, 929 (2012).
T. Swain, J. Therm. Anal. Calorim. 103, 1111 (2011).
J.J. Biendicho, K.-C. Haiso, S. Hull, and A.R. West, Inorg. Chem. 56, 3657 (2017).
A. Omek, Chem. Eng. J. 331, 501 (2018).
C.M. Julien and M. Massot, in, Proceedings of the International Workshop on Advanced Techniques for Energy Sources Investigation and Testing (Sofia, 2004). pp L3-1-L3-17.
A. Periasamy, S. Muruganand, and M. Palaniswamy, Rasayan J. Chem. 2, 981 (2009).
X.-Z. Chen, J.-H. Tang, Y.-N. Cui, M. Liu, and D. Mao, J. Chem. Eng. Data. 59, 481 (2014).
P. Porkodi, V. Yegnaraman, P. Kamaraj, V. Kalyanavalli, and D. Jeyakumar, Chem. Mater. 20, 6410 (2008).
G.K. Willianson and W.H. Hall, Acta Metall. 1, 22 (1953).
U. Thupakula, A. Jena, A.H. Khan, A. Dalui, and S. Acharya, J. Nanopart. Res. 14, 701 (2012).
A. Ghule, R. Murugan, and H. Chang, Thermochim. Acta 371, 127 (2001).
S.J. Milne and A.R. West, J. Solid State Chem. 57, 166 (1985).
M. Kizilyalli and A.J.E. Welch, J. Inorg. Nucl. Chem. 38, 1237 (1976).
A. Ghule, N. Baskaran, R. Murugan, and H. Chang, Solid State Ionics 161, 291 (2003).
A. Abhat, Sol. Energy 30, 313 (1983).
G. Lane, Latent Heat Materials, vol. 1 (Boca Raton: CRC Press, 1983).
H.P. Garg, S.C. Mullick, and A.K. Bhargava, Solar Thermal Energy Storage (Dordrecht: D. Reidel, 1985).
B. Zalba, J.M. Marin, L.F. Cabeza, and H. Mehling, Appl. Therm. Eng. 23, 251 (2003).
Y. Huang, Y.-C. Lin, D.M. Jenkins, N.A. Chernova, Y. Chung, B. Radhakrishnan, I.-H. Chu, J. Fang, Q. Wang, F. Omenya, S.P. Ong, and M.S. Whittingham, ACS Appl. Mater. Interfaces 8, 7013 (2016).
G.M. Nolis, F. Omenya, R. Zhang, B. Fang, S. Upreti, N.A. Chernova, F. Wang, J. Graetz, Y.-Y. Hu, C.P. Grey, and M.S. Whittingham, J. Mater. Chem. 22, 20482 (2012).
P. Hanggi and G.-L. Ingold, Acta Phys. Pol. B 37, 1537 (2006).
P. Hanggi, G.-L. Ingold, and P. Talkner, N. J. Phys. 10, 115008 (2008). https://doi.org/10.1088/1367-2630/10/11/115008.
A.J. Leggett, S. Chakravarty, A.T. Dorsey, M.P.A. Fisher, A. Garg, and W. Zwerger, Rev. Mod. Phys. 59, 1 (1987).
Acknowledgements
The author thanks the Indic Institute of Design and Research for providing necessary laboratory facilities for synthesis of this compound. G.S.B. is grateful to the Director, FST, IFHE for granting permission to become associated with this work. T.S. thanks ST&IC, Cochin University of Science and Technology, Cochin for sophisticated measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Swain, T., Brahma, G.S. Synthesis, Characterization, and Thermal Behavior of Ni3(PO4)2·8H2O·Na3PO4·3.5H2O·0.75Na2SO4. J. Electron. Mater. 47, 2817–2823 (2018). https://doi.org/10.1007/s11664-018-6132-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-018-6132-x