Abstract
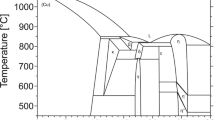
The phase boundaries of the Ag-In binary system were determined by the diffusion couple method, differential scanning calorimetry (DSC) and metallographic techniques. The results show that the region of the (hcp) phase is narrower than that reported previously. Thermodynamic calculation of the Ag-In system is presented by taking into account the experimental results obtained by the present and previous works, including the data on the phase equilibria and thermochemical properties. The Gibbs energies of liquid and solid solution phases are described on the basis of the sub-regular solution model, and that of the intermetallic compounds are based on the two-sublattices model. A consistent set of thermodynamic parameters has been optimized for describing the Gibbs energy of each phase, which leads to a good fit between calculated and experimental results. The maximum bubble pressure method has been used to measure the surface tension and densities of liquid In, Ag, and five binary alloys in the temperature range from 227°C to about 1170°C. ON the basis of the thermodynamic parameters of the liquid phase obtained by the present optimization, the surface tensions are calculated using Butler’s model. It is shown that the calculated values of the surface tensions are in fair agreement with the experimental data.
Similar content being viewed by others
References
M.R. Baren, Indium Alloys and their Engineering Applications, ed. C.E.T. White and H. Okamoto (Materials Park, OH: ASM Intl., 1993), p. 15.
F. Weibke, A. Anorg. Chem. 222, 145 (1935).
E. Hellner, Z. Metallkd. 42, 17 (1951).
L.L. Frevel and E. Ott, J. Am. Chem. Soc. 57, 228 (1935).
A.N. Campbell, Can. J. Chem. 48, 1703 (1970).
O.J. Kleppa, J. Phys. Chem. 60, 846 (1956).
L.R. Orr and R. Hultgren, J. Phys. Chem. 65, 378 (1961).
T. Nozaki, Mater. Trans. JIM 7, 52 (1966).
R. Beja, C.R. Acad. Sci. 267C, 123 (1968).
C.B. Alcock, Acta Metall. 17, 839 (1969).
R. Castanet, J. Chim. Phys. 67, 789 (1970).
B. Predel and U. Schallner, Z. Metallkd. 63, 341 (1972).
D.B. Masson, Metall. Trans. A 4A, 991 (1973).
C.B. Alcock, Acta Metall. 21, 1003 (1973).
K. Kameda, J. Japan Inst. Metals 45, 614 (1981).
G. Qi, Mater. Trans. JIM 30, 75 (1989).
M. Bienzle and F. Sommer, Z. Metallkd. 85, 766 (1994).
T.M. Kornenhen and J.K. Kivilahti, J. Electron. Mater. 27, 149 (1998).
J.A.V. Butler, Proc. Roy. Soc. 135A, 348 (1932).
H. Enoki, K. Ishida, and T. Nishizawa, J. Less-Common Met. 160, 153 (1990).
S. Sugden, J. Chem. Soc. 124, 27 (1924).
F. Bashforth and J.C. Adams, An Attempt to Test the Theory of Capillary Action, Cambridge, U.K.: Cambridge Univ. Press, 1883).
O. Redlich and A.T. Kister, Ind. Eng. Chem. 24, 345 (1948).
A.T. Dinsdale, CALPHAD 15, 317 (1991).
T. Tanaka, K. Hack, T. Iida, and S. Hara, Z. Metallkd. 87, 380 (1996).
B. Sundaman, B. Jansson, and J.O. Andersson, CALPHAD 9, 153 (1985).
W. Huma-Rothery, Phil. Trans. Royal Soc. London, Ser. A 233, 1 (1936).
E.A. Owen, Phil. Mag. 27, 294 (1939).
M.E. Staumanis, Trans. Metall. Soc. London AIME 233, 964 (1956).
E.E. Libman, Bull. Ill. Univ. Eng. Exp. Sta. 187 (1928).
W.D. Kingery and M. Humenik, J. Phys. Chem. 57, 358 (1953).
I. Lauerman, G. Metzger, and F. Sauerwald, Z. Phys. Chem. Bol 216, 1 (1960).
S.K. Rhee, J. Am. Ceram. Soc. 53, 639 (1970).
G. Bernard and C.H.P. Lupis, Metall. Trans. 2, 555 (1971).
A. Kasama, T. Iida, and Z. Morita, J. Jpn. Inst. Met. 40, 1030 (1976).
M. Brunet, J.C. Joud, N. Eustathopoulos, and P. Derse, J. Less Common Met. 51, 59 (1977).
R. Sengiorgi, M.L. Muolo, and A. Passerone, Acta Metall. 30, 1597 (1982).
K. Nogi, K. Oishi, and K. Ogino, Mater. Trans. JIM 30, 137 (1989).
I. Egry, G. Lohófer, and S. Schneider, Paper presented at the 10th Int. IUPAC Conf. High Temperature Materials Chemistry (Julich, Germany, 10–14 April 2000), p. 87.
D.A. Melford and T.P. Hoar, J. Inst. Met. 85, 197 (1956–57).
S.P. Yatsenko, W.I. Kononenko, and A.L. Schukman, Teplofiz. Vys. Temp. 10, 66 (1972).
G. Lang, J. Inst. Met. 101, 300 (1973).
G. Lang, P. Laty, J.C. Joud, and P. Desre, Z. Metallkd. 68, 133 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moser, Z., Gasior, W., Pstrus, J. et al. Studies of the Ag-In phase diagram and surface tension measurements. J. Electron. Mater. 30, 1120–1128 (2001). https://doi.org/10.1007/s11664-001-0138-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11664-001-0138-4