Abstract
Summary
The IOF Epidemiology and Quality of Life Working Group has reviewed the potential role of population screening for high hip fracture risk against well-established criteria. The report concludes that such an approach should strongly be considered in many health care systems to reduce the burden of hip fractures.
Introduction
The burden of long-term osteoporosis management falls on primary care in most healthcare systems. However, a wide and stable treatment gap exists in many such settings; most of which appears to be secondary to a lack of awareness of fracture risk. Screening is a public health measure for the purpose of identifying individuals who are likely to benefit from further investigations and/or treatment to reduce the risk of a disease or its complications. The purpose of this report was to review the evidence for a potential screening programme to identify postmenopausal women at increased risk of hip fracture.
Methods
The approach took well-established criteria for the development of a screening program, adapted by the UK National Screening Committee, and sought the opinion of 20 members of the International Osteoporosis Foundation’s Working Group on Epidemiology and Quality of Life as to whether each criterion was met (yes, partial or no). For each criterion, the evidence base was then reviewed and summarized.
Results and Conclusion
The report concludes that evidence supports the proposal that screening for high fracture risk in primary care should strongly be considered for incorporation into many health care systems to reduce the burden of fractures, particularly hip fractures. The key remaining hurdles to overcome are engagement with primary care healthcare professionals, and the implementation of systems that facilitate and maintain the screening program.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In most countries, screening is regarded as a public health measure, the purpose of which is to offer a test to identify those individuals who are more likely to benefit from further investigations and/or treatment to reduce the risk of a disease or its complications. The test is targeted at a defined population, the members of which do not necessarily perceive that they are at risk of, or are already affected by, the disease or its complications. Principles to determine whether the approaches to managing a disease should include a screening programme were first proposed over 50 years ago [1] (Table 1).
These principles have remained largely intact since then, with some modification by national and regional screening committees. For example, prior to making a formal assessment of a screening program, the UK National Screening Committee (NSC) examines certain general characteristics, unrelated to the specific disease, to guide decision making (Table 5). These include that the target population to be screened should be sufficiently large to enable safe, clinically and cost-effective screening, and that the population to be screened would regard themselves as not necessarily having symptoms of the disease or to be at risk of the disease (i.e. relatively healthy people). There should exist an effective means of identifying and contacting the whole cohort to be offered screening, including proactive approaches (e.g. written invitation, verbal invitation at other appointments), with those approached properly informed of the potential benefits and risks to make an informed choice. If these characteristics can be satisfied, the subsequent formal assessment of the evidence for screening covers the key issues, identified by Wilson and Jungner, relating to the condition, the test, the treatment and the effectiveness of any proposed screening programme. Several further aspects, considered by the UK NSC, are beyond the scope of this report: these include plans for managing and monitoring the screening programme, agreed quality assurance standards, adequate levels of staffing and facilities, provision of information to potential participants and anticipated public pressure to extend the eligibility criteria for screening.
As described below, osteoporosis and, more pertinently, its burden of fractures represents an opportunity for consideration of a screening strategy. The purpose of this paper is to propose a screening strategy for fracture risk reduction in postmenopausal women and to examine key issues in relation to this strategy.
The proposed screening strategy
Given the effectiveness and cost-effectiveness of the intervention described later in this document, the strategy is based on the approach undertaken in the Screening for Osteoporosis in Older People (SCOOP) study in the UK [2,3,4,5,6]. In brief, a risk factor questionnaire based on the FRAX® risk assessment tool would be completed, in paper form or electronically, by women age 70 years or older through self-completion or completion assisted by a family member or caregiver. The questionnaire data would then be utilized centrally to calculate the 10-year major osteoporotic fracture probability and the 10-year hip fracture probability. Those with a low risk of hip fracture would receive a letter of reassurance with general lifestyle advice, while the remainder would have an additional assessment of femoral neck bone mineral density (BMD) using local densitometer facilities. The bone density result would then be incorporated in an updated FRAX calculation, with those that have hip fracture probabilities above the intervention threshold being recommended for treatment. The latter recommendation would be communicated to both the individual and their general practitioner.
As the effectiveness of any screening programme is not only dependent on the screening test but also on the prevalence of the disease of interest, we envisage that the strategy should initially be considered in older age groups, for example those age 70 years and above as in the SCOOP trial, in those countries with a hip fracture risk comparable to or higher than that in the UK [7].
Methods — survey and responses
The opinion of 29 individual expert members of the Epidemiology and Quality of Life (EpiQOL) Working Group on whether screening for high hip fracture risk could fulfil each of the UK NSC criteria (Table 5) for a screening programme was canvassed using a Google Form. The survey was limited to the first 15 criteria as the final four address criteria that focus on issues around implementation of an accepted screening strategy. For each criterion, the experts were asked to say if the proposed strategy fulfilled that criterion (yes, in part or no) and could expand on their classification in a free text field.
Responses were received from 20 experts (69% of those approached), and the overall scores for each criterion are included in Table 5. Their feedback was incorporated into each of the sections below.
Results-Criterion review
The condition
The condition sought should be an important health problem
Osteoporosis is a systemic skeletal disease, characterised by low bone mass and microarchitectural deterioration of bone tissue with a subsequent increase in bone fragility and susceptibility to fracture [8]. The most serious clinical consequence of osteoporosis is hip fracture, though other fractures commonly occur at the spine, forearm and shoulder, and are often grouped together with hip fractures as major osteoporotic fractures.
Osteoporotic fractures are undoubtedly a common public health problem, particularly in ageing societies in terms of patient’s health, quality of life and social care costs [9,10,11,12]. At the age of 50 years, the remaining lifetime probability of at least one of the major osteoporotic fractures is 22% in men and 46% in women. In 2000, it was estimated that there were approximately 9 million fractures annually worldwide, with over one-third of all osteoporotic fractures occurring in Europe [13]. The latter accounted for 2 million disability-adjusted life years (DALYs) annually in Europe, a burden that exceeded that of hypertensive heart disease or rheumatoid arthritis. The number and burden of osteoporotic fractures is rising in many countries, partly related to the increased longevity of the populations. The age- and sex-specific incidence of fracture has also increased in some but not all countries [14, 15].
In 2019, 4.3 million new fragility fractures were estimated to have occurred in the EU, comprising approximately 827,000 hip fractures, 663,000 clinical vertebral (spine) fractures, 637,000 forearm fractures and 2,150,000 fractures at other sites (i.e. pelvis, rib, humerus, tibia, fibula, clavicle, scapula, sternum and other femoral fractures) [11]. About two-thirds of all incident fractures occurred in women. In 2019, the number of deaths causally related to fractures was estimated to be 248,487, with about half of these attributable to hip fractures. The number of fracture-related deaths was comparable or exceeded those from some of the most common causes of death such as lung cancer, diabetes and chronic lower respiratory diseases. In 2000, it was estimated that osteoporotic fractures in Europe accounted for more DALYs (2,006,000) than rheumatoid arthritis (1,048,000), but less than for osteoarthritis (3,088,000) [13]. In 2016, the estimate placed the total DALY loss related to fragility fractures at more than 2.6 million, which was higher than that estimated for chronic obstructive pulmonary disease and ischaemic stroke, but lower than that for lung cancer or dementia [12].
In 2019, the cost of osteoporosis, including pharmacological intervention in the EU was estimated at €56.9 billion (1000 million), with two-thirds derived from the treatment of fractures and only 3% representing the costs of pharmacological intervention. Excluding the latter cost, hip fractures represented 54% of the costs. The total cost including values of quality adjusted life years (QALYs) lost was estimated at €113 billion, a figure that is expected to rise to €121 billion in 2025. The cost of osteoporotic fractures accounts for approximately 3.5% of healthcare spending (i.e. €55.3 billion in 2019) indicating a very substantial impact of fragility fractures on the present healthcare budgets of European countries.
The epidemiology of osteoporosis is well characterized and described in detail elsewhere [14, 16,17,18,19]. The incidence of fragility fractures increases markedly with age, and the vast majority of osteoporotic fractures occur in older women. Compared with other fractures, a great deal of information is available on the epidemiology of hip fracture as nearly all such patients are admitted to hospital with data available through surgical and discharge records. In patients with fractures at other skeletal sites, only a minority are admitted, though they may attend hospital on an outpatient basis.
The occurrence of hip fracture, widely regarded as the most serious osteoporotic fracture, reflects decreasing bone strength with increasing age, with a concomitant increase in falls risk [20]. The risk of falling is somewhat higher in elderly women than in elderly men with about one-third of elderly individuals falling annually [21]. Hip fractures are relatively rare at the age of 50 years but become the predominant fracture from the age of 75 years [22]. Patients with hip fracture often have significant co-morbidities, and up to 20% of patients die in the first year following hip fracture, mostly as a result of these underlying medical conditions [23, 24]; nonetheless, it is estimated that approximately 30% of deaths are causally related to the fracture event [25].
In summary, there is ample evidence that osteoporosis and, in particular, osteoporosis-related fractures are a significant and growing health problem for individuals and healthcare providers. Fractures are highly prevalent and are associated with significant morbidity and mortality.
The epidemiology and natural history of the condition, including development from latent to declared disease, should be adequately understood and there should be a detectable risk factor, disease marker, latent period or early symptomatic stage
Clinically, bone health is most widely assessed by the non-invasive measurement of BMD by dual-energy x-ray absorptiometry (DXA) as there is a very well-established inverse correlation between BMD and future fracture risk [26]. As BMD is continuously distributed in the population, a WHO Scientific Group in 1994 developed a densitometric definition of osteoporosis using a threshold of 2.5 standard deviations below the mean BMD in young people of the same sex [27, 28]. Since then, osteoporosis has been operationally defined on the basis of BMD assessment, with a subsequent refinement to focus on measurements at the femoral neck [29]. This definition, however, has high specificity but low sensitivity for the prediction of fracture, as the majority of osteoporotic fractures occur in individuals with BMD values above the osteoporosis threshold [27]. This is the main reason that BMD measurement alone has not been accepted as a public health screening test in many countries to date, though there are notable exceptions including the USA [30, 31].
As a clinical endpoint, osteoporotic fracture has multiple factors contributing to the risk of fracture that can enable identification of high risk prior to the event occurring. In many ways, this is a different latency to that identified under established screening programmes in diseases such as breast and cervical cancer or diabetic eye disease, where the focus is on early detection of the disease itself. At a simplistic level, it might be assumed that low or decreasing bone mass is the only latent stage of note for osteoporotic fracture but, as described below, this excludes a wealth of information on other factors that contribute to fracture risk. This awareness has led to the development of risk calculators that take account of several risk indicators as used in the management of other diseases such as cardiovascular disease [32,33,34].
Examples of ‘non-BMD’ factors that contribute to fracture risk include ethnicity, age, sex, body mass index (BMI) [35], a prior fracture [36], a family history of fracture [37], long-term use of systemic glucocorticoids [38], a history of rheumatoid arthritis [39], diabetes [40,41,42], other causes of secondary osteoporosis [43] and lifestyle risk factors such as physical inactivity [44], falls [45, 46], smoking [47] and alcohol consumption[48]. It is important to note that some of these risk factors are partially or wholly independent of BMD, so that their use with BMD can enhance the information provided by BMD alone. Conversely, some strong BMD-dependent risk factors, for example BMI, can be used for fracture risk assessment in the absence of access to BMD tests. This is discussed in more detail in the section on the test.
In summary, the epidemiology and natural history of osteoporosis, including the risk factors that contribute to the clinical endpoint of fracture, are well documented and understood. The recognition of identifiable risk factors and the quantitation of that risk are now possible allowing preventative interventions to be targeted to those at highest fracture risk.
All the cost-effective primary prevention interventions should have been implemented as far as practicable
To date, there is no evidence base to demonstrate that cost-effective (and indeed effective) primary prevention interventions of a non-pharmacological nature reduce the burden of osteoporotic fractures. Where appropriate, lifestyle modifications are recommended comprising advice about nutrition (adequate calcium, vitamin D and protein intake), physical activity (weight-bearing, muscle strengthening and balance training exercise) and risk factor reduction (e.g. smoking cessation, alcohol intake reduction, vision correction, avoidance or reduction in exposure to drugs associated with osteoporosis). In general, a daily dietary calcium intake of around 800–1200 mg is recommended, with calcium supplementation where this cannot be achieved [49,50,51,52,53,54]. National recommendations for vitamin D supplementation vary according to the target population (whole population or subsets), the required dose and the target level of vitamin D as assessed by serum or plasma 25-hydroxyvitamin D [55,56,57,58]. The impact of combined calcium and vitamin D supplementation on fracture risk remains uncertain, particularly in the community-dwelling elderly [59,60,61].
Thus, whereas patients will continue to receive general advice in the absence of cost-effective primary prevention interventions, there remains a need to implement other proven, cost-effective strategies and interventions.
The test
There should be a simple, safe, precise and validated screening test
The increasing recognition and acceptance that treatment for osteoporosis should be targeted on the basis of fracture risk requires well-validated assessment tools providing ease of use in clinical practice. Over the last 15 years, three tools have garnered interest, namely the FRAX® tool [62, 63], QFracture [64, 65] and the Garvan tool [66]. Of these, the FRAX tool has achieved widespread use with incorporation into numerous guidelines worldwide, with a large number of studies evaluating its utility [67]. In contrast to many risk calculators, both in the field of fracture risk or other disease areas, FRAX is unusual in that the output from the calculation is calibrated to the epidemiology of fracture and mortality for each country/region/ethnicity with an existing FRAX model. In short, this means that if the whole population were to undergo FRAX assessments, then the number of fractures predicted would be the number observed. This construct has been used to develop simulated population cohorts in the UK and elsewhere and has provided a basis for estimating the global burden of high fracture probabilities [18, 68, 69]. Most importantly, in the context of this review, FRAX is the only fracture risk assessment tool to be studied to date in randomised, controlled studies of population-based screening.
In brief, the FRAX® tool (https://www.sheffield.ac.uk/FRAX/index.aspx) is a computer-based algorithm developed by researchers at the then WHO Collaborating Centre for Metabolic Bone Diseases at the University of Sheffield [43, 70, 71]. The calculation incorporates information gathered from easily obtained clinical risk factors (Fig. 1) to estimate the probability of sustaining a fracture in the next 10 years. Femoral neck BMD can also be added to the calculation to enhance fracture risk prediction. It has two outputs, namely the probability of sustaining a hip fracture in the next 10 years, or a major osteoporotic fracture. The outputs are calibrated to age and sex-specific rates of fracture and mortality in a substantial number of countries/territories (currently 85 models covering 77 countries).
FRAX has been widely used for the assessment of fracture risk since the launch of the FRAX website in 2008. Following regulatory review by the US Food and Drug Administration, FRAX was incorporated into DXA scanners to provide FRAX probabilities at the time of DXA scanning. For those without internet access, hand-held calculators and an application for Apple smartphones have been developed. A paper-based FRAX pad in several languages allows patients to document risk variables prior to a visit with healthcare professionals.
It is also worthy of note that the availability and access to densitometry in many countries is low [72,73,74,75], so that a major advantage of FRAX is the ability to assess fracture risk where BMD is unavailable. In the UK, National Institute for Health and Care Excellence (NICE) has recommended that FRAX (or QFracture) should be used to estimate 10-year predicted absolute fracture risk prior to deciding on the need for DXA measured BMD [76]. As a completely non-invasive test, the tool is safe for users.
The characteristic of major importance, for the purpose of risk assessment, is the ability of a tool to predict the occurrence of new fractures, more usefully expressed as the increase in relative risk per standard deviation (SD) unit increase in risk score, termed the gradient of risk. The gradient of risk for the use of the FRAX clinical risk factors alone, femoral neck BMD alone, and the combination is shown in Table 2 [77]. It is relevant that the performance of FRAX is enhanced by the use of BMD tests; FRAX without BMD has a predictive value for fractures that is comparable to the use of BMD alone. Overall, the predictive value compares very favourably with other risk engines such as the Gail score for breast cancer [78]. The choice of screening modality to identify high hip fracture risk can be adapted to local resources; for example, where BMD measurements are readily available, it can play a predominant role in screening, while screening with FRAX risk factors alone can readily be applied in the absence of access to BMD.
The performance characteristics of the FRAX clinical risk factors, with and without BMD, have been validated in eleven independent population-based cohorts [77]. Notably, the gradients of risk and age-adjusted area under the curves (AUC) from receiver-operated characteristic (ROC) analysis were comparable in the validation cohorts compared with the original cohorts. In contrast to separate analyses of sensitivity and specificity, the AUC provides an index of overall test accuracy if sensitivity and specificity have equal weights. A test with no predictive (or discriminative) ability would have an AUC of 0.5 (or 50%), while a perfect predictive test would have an AUC of 1.0 (or 100%). For hip fracture prediction without BMD, the mean AUC at age 70 years was 0.66 in the validation cohorts compared with 0.67 in the original cohorts. With the addition of BMD, the mean AUC was 0.74 and 0.78, respectively [77]. Since that original validation, a number of independent reviews, including four systematic reviews, have included detailed descriptions of the performance of the FRAX tool [76, 79,80,81,82]. Bearing in mind the flaws that can arise from cross-study and within-study comparisons of the AUC [83, 84], the reviews have confirmed a significant predictive ability of FRAX for future fractures, especially of the hip. For example, in the most recent of the systematic reviews, conducted for the US Preventive Services Task Force, the AUCs for hip fracture prediction with FRAX ranged from 0.76, in 12 studies comprising just under 200,000 women where FRAX was calculated without BMD, to 0.79 in 10 studies comprising approximately 162,000 women when FRAX was calculated with BMD [79]. In a recent independent comparative study in a single population sample, the predictive value for hip fractures was better for the FRAX tool (AUC: 0.841, 95% CI 0.795–0.887) than for the Garvan fracture risk tool (AUC: 0.769, 95% CI 0.702–0.836, p = 0.01) [85].
In summary, the FRAX tool fulfils the criteria of being a simple, safe, precise and well-validated test. While the AUCs for future hip fracture prediction are perhaps lower than existing screening tests that detect existing or early disease, they suggest excellent performance suitable for application in clinical practice.
The distribution of test values in the target population should be known and a suitable cut-off level defined and agreed.
Screening programmes are usually designed and adopted at regional or national levels. The same is true for the development and setting of intervention thresholds; this necessarily remains a more localised remit, given that each health care system will consider local/national factors such as reimbursement issues, health economic assessment, willingness to pay for health care in osteoporosis and access to DXA. The use of health economic analyses to derive intervention thresholds is fraught with problems, the most common of which is that they are time limited due to changes, usually reductions, in the costs of treatment. In the presence of very inexpensive medications, absurd situations can arise with a recent example from the UK where, following a multiple technology appraisal (MTA) on bisphosphonate use in osteoporosis, NICE recommended that treatment with oral bisphosphonates may be instituted in those with a 10-year probability of major osteoporotic fracture of 1% or more [86]. If interpreted as an intervention threshold, virtually all women aged ≥ 65 and men ≥ 75 years would be recommended treatment [87]. Shortly thereafter, NICE endorsed the assessment and intervention thresholds proposed within the NOGG guidance [50, 88].
A systematic review in 2016 identified assessment guidelines for osteoporosis that incorporated FRAX, utilising either age-independent (i.e. fixed) or age-dependent thresholds for further assessment and/or treatment [67]. In most guidelines, treatment for osteoporosis is recommended in individuals with prior fragility fractures, especially fractures at the spine or hip, with the FRAX thresholds reserved specifically for use in those without such fractures. The majority of guidelines had utilised a fixed intervention threshold, frequently as a component of more complex guidance (e.g. BMD thresholds). A substantial proportion (about 25%) had adopted an age-dependent threshold, with a small minority proposing a combination of age-dependent and fixed thresholds (so called hybrid thresholds).
Age-dependent intervention thresholds, first developed by the National Osteoporosis Guideline Group (NOGG), are based on the rationale that if a woman with a prior fragility fracture is eligible for treatment, then, at any given age, a man or woman with the same fracture probability (i.e. at the ‘fracture threshold’) should also be eligible, even in the absence of fracture [50, 89, 90]. By design, this fracture threshold increases with age with a plateau or decline at older ages due to the competing hazard of death. Age-dependent thresholds have since been adopted into European guidelines [51, 91] and elsewhere [67, 92, 93]. The same intervention threshold is applied to men, since the effectiveness and cost-effectiveness of interventions in men are broadly similar to that in women for equivalent risk [94]. In the UK, it was noted that the age-dependent probability thresholds introduced inequalities in access to therapy at older ages (≥ 70 years) for women without a prior fracture, leading to the development of a hybrid model which reduced the disparity [69]. For the proposed screening strategy, the approach would be to adopt the current NOGG thresholds in the UK. The thresholds at age 70 years for major osteoporotic fracture and hip fracture in the UK and some other high-risk countries, if using a similar approach, are shown in Table 3. Other countries that have an average 10-year hip fracture probability threshold of 5% or more include Austria, Denmark, Germany, Greece, Iceland, Iran, Ireland, Israel, Italy, Kazakhstan, Kyrgystan, Malta, Moldova, Norway, Singapore, Slovakia, South Korea, Sweden, Switzerland, Taiwan and Uzbekistan.
In summary, there are a number of approaches to defining cut-off levels for the FRAX tool as intervention thresholds within a screening program. Of these, the probability thresholds at age 70 in women with a prior fracture seem intuitive, as all current guidelines would recommend treatment in such individuals.
The test should be acceptable to the population
The field of research into acceptability of healthcare interventions is, perhaps surprisingly, underdeveloped [95]. DXA-based measurement of BMD is a noninvasive procedure, and the level of radiation exposure is very low and considered to be safe for the population [96]. Access to, and use of, the FRAX online tool suggests that it is viewed to have acceptable clinical utility by health professionals [67, 97, 98], though this has not been formally assessed. In the setting of primary prevention, a formal assessment has been undertaken as part of the SCOOP study [2]. This qualitative sub-study sought to capture the views of older women and GPs about the acceptability of screening, using the FRAX questionnaire with BMD in those at medium to high risk, to prevent fractures [99]. The women and GPs were found to view screening positively. Risk assessment using the FRAX tool, compared to usual care, showed no impact on anxiety (State-Trait Anxiety Inventory) and quality of life (EuroQol 5-Dimension tool and the Short-Form Health Survey 12) (P > 0.10 for all outcomes). The authors concluded that an effective and cost-effective screening programme to reduce osteoporotic fractures could be implemented in routine care and would be well received by women and GPs [99].
The data to date suggest that assessment of fracture risk by the FRAX tool, with subsequent BMD measurement where indicated, is acceptable to the populations in which it has been used.
There should be an agreed policy on the further diagnostic investigation of individuals with a positive test result and on the choices available to those individuals.
The use of risk calculators to determine who should receive an intervention is somewhat different from many established screening programmes. For example, breast tissue calcification might indicate malignancy, but further investigation is required to confirm or refute the diagnosis of breast cancer. In contrast, the patient identified at high risk by a risk calculator arrives there because of the known factors entered into the calculation. In many osteoporosis guidelines, this risk assessment may indicate the need for BMD measurement, if not already undertaken, to provide additional risk information.
In clinical settings, this assessment of BMD, usually by DXA measurements at the lumbar spine and hip, is used not just for risk prediction, but also for diagnosis, selection of patients for treatment and monitoring of patients on treatment. A requirement has been estimated of approximately 11 DXA units per million of the general population to permit implementation of practice guidelines [72], a level which is only achieved in about 60% of countries in the EU [11, 100]. Strategies that target the use of DXA to those with fracture risks at or near an intervention threshold reduce the need for DXA while maintaining the identification of those at high risk. For example, a comparison of the NOGG strategy, comprising risk assessment followed by targeted DXA, with the previous guidance issued by the Royal College of Physicians (RCP) where the presence of a risk factor simply mandated the use of DXA, NOGG identified a similar proportion of women at high risk (average 34.6% vs. 35.7% across all ages), but with lower numbers of scans required at each age [68]. Thus, the NOGG approach required only 3.5 scans at the age of 50 years to identify one case of hip fracture, whereas the previous RCP approach required 13.9. At 75 years, the corresponding numbers were 0.9 and 1.5. Compared to the older strategy, the FRAX-based NOGG strategy used BMD resources more efficiently with lower acquisition costs and lower costs per hip fracture averted [68].
Most clinical guidelines agreement the need for additional radiological, haematological, biochemical and immunological assessments in patients at high risk of fracture or with recently diagnosed osteoporosis [50, 51, 53]. Broadly, these seek to identify or exclude any undetected underlying disease that might contribute further to fracture risk and its management (e.g. identify a reversible or treatable cause such as primary hyperparathyroidism or coeliac disease), and to identify any contraindications to a particular treatment (e.g. renal impairment in the case of bisphosphonates or hypocalcaemia in the use of potent bisphosphonates or denosumab).
In summary, there is much agreement across clinical guidelines on the further diagnostic investigation of individuals at increased risk of fracture and on the treatment choices available to such individuals (see next section).
The treatment
There should be an effective treatment or intervention for patients identified through early detection, with evidence of early treatment leading to better outcomes than late treatment
Approaches to reduce fracture risk incorporate non-pharmacological measures [101, 102] and pharmacological treatments. Of the many factors that influence the risk of fracture, age-related reductions in bone mass and increased likelihood of falling are important contributors [103]. While assessment of falls risk and appropriate interventions aimed at reducing falls risk have been shown to be effective [104], at least in the short term, their impact on the risk of fracture, particularly at the hip, is less certain [105, 106]. For example, in a recent review, multifactorial interventions or single exercise-based interventions were found to reduce falls risk, but the impact on fractures was not significant [107].
In contrast, many randomised, placebo-controlled trials have shown that treatments directed at preventing bone loss and/or improving bone mass can significantly reduce the incidence of fracture [108] at vertebral (relative risk, RR 0.4–0.6) and non-vertebral sites (RR 0.6–0.8), including reducing the incidence of hip fracture by 30–45% [109,110,111]. Classes of drugs demonstrating efficacy in the prevention of fragility fracture include bisphosphonates (e.g. alendronate, ibandronate, risedronate and zoledronate), selective oestrogen receptor modulators [SERM] (e.g. raloxifene, bazedoxifene), parathyroid hormone peptides and derivatives (e.g. teriparatide and abaloparatide), menopausal hormone therapy (MHT) and humanized monoclonal antibodies (e.g. denosumab and romosozumab). Recent comparative clinical trials have provided evidence of enhanced anti-fracture efficacy of anabolic compared with antiresorptive therapies [112,113,114], prompting considerations of starting treatment with an anabolic agent in patients at very high risk of fracture as a more appropriate means of rapidly reducing fracture risk [115,116,117].
The availability of effective therapies has long given rise to considerations of approaches for fracture prevention; for example, almost 20 years ago, a simulation model investigated the cost-effectiveness of a 5-year treatment to prevent hip fracture in women at average risk [118]. Major determinants of cost-effectiveness included the age of the individual and the cost of treatment. Assuming an efficacy of a 35% reduction in relative risk, it was cost-effective to treat all women above the age of 81 years with an intervention cost of $650/year. For cheaper treatments, e.g. $250, it was cost-effective to give treatments at the age of 70 years, and for the cheapest treatment to treat nearly all postmenopausal women. The authors suggested that controlled prospective studies in the apparently healthy population were worthy of consideration, particularly in the elderly. Since then, several randomised, controlled studies have examined the use of osteoporosis interventions in older populations, unselected for osteoporosis. In the Women’s Health Initiative study, the use of MHT was examined in two studies, one in women with an intact uterus and one in women with a history of hysterectomy [119, 120]. The effects on fracture risk were similar between the two studies. In women age 50–79 years with an intact uterus, the use of conjugated equine oestrogen and medroxyprogesterone acetate over a mean of 5.2 years follow-up reduced the incidence of hip fracture by 34% (hazard ratio, HR: 0.66; 95% CI: 0.45–0.98], with a 23% reduction in other osteoporotic fractures (HR: 0.77; 95% CI: 0.69–0.86) [119]. Importantly, this effect was independent of baseline BMD [121] suggesting that treatment efficacy was not dependent on the presence of osteoporosis. A similar BMD-independent effect on fracture risk reduction was observed in a 3-year randomised, placebo-controlled study of the oral bisphosphonate, clodronate, in women age 75 years and older, again unselected for osteoporosis [122]. This study was underpowered for the outcome of hip fractures, but treatment was associated with a 23% reduction in osteoporotic fractures. A post hoc analysis demonstrated that, while the effect was independent of BMD, treatment was more effective in women at higher baseline fracture risk assessed by the FRAX tool [123]. Thus, at a probability of 15% (25th percentile), the relative risk for fracture was reduced by 8% (RR 0.92, 0.69–1.24), whereas at probabilities of 24% and 30% (the 75th and 90th percentiles), the reductions were 27% (RR 0.73, 0.58–0.92) and 38% (HR 0.62, 0.46–0.84) respectively. Several other studies of a variety of osteoporosis therapies have also shown no interaction between treatment efficacy and underlying BMD [124] supporting the use of treatment in those with high fracture risk even before a BMD in the osteoporosis range has been reached.
The success of such population-based approaches does not necessarily imply that risk assessments to target therapies are not worthwhile, since it is likely to be even more cost-effective to target individuals at higher-than-average risk. Certainly, the more expensive the treatment and the cheaper the risk assessment, the stronger the case for selecting high-risk segments of the population. The age at which intervention might be offered depends critically upon the costs of treatment, efficacy and the average risk of hip fracture, as well as additional skeletal and extraskeletal effects. The aforementioned cost-effectiveness analysis in 2001 suggested that strategies aimed at intervention over the age of 70 years required testing [118].
In summary, there is an extensive clinical and economic evidence base for effective bone-targeted treatments that can reduce fracture risk. Unselected population-based studies suggest that fracture risk can be also significantly reduced with such treatments; targeted intervention of relatively inexpensive, safe treatments to those within these populations that have identifiable increased risk will intuitively lead to better outcomes than treatment delayed until after the subsequent occurrence of fracture.
There should be agreed evidence-based policies covering which individuals should be offered treatment and the appropriate treatment to be offered
Most clinical guidelines for the management of osteoporosis adopt a case-finding strategy, combining clinical risk factors with measurement of BMD, preferably at the hip, to assess fracture risk and subsequent use of defined intervention thresholds [67]. The majority recommend treatment for osteoporosis in individuals with prior fragility fractures, especially fractures of the spine or hip, without specific need for BMD assessment. For those without prior fractures, intervention thresholds have historically focused on the BMD T score ≤ − 2.5, with treatments reimbursed in many healthcare settings when a BMD-based diagnosis of osteoporosis is confirmed. As noted previously, the realization that BMD-defined osteoporosis has high specificity, but low sensitivity for future fractures has underpinned the movement towards the use of absolute fracture risk to define intervention thresholds, usually calculated by the FRAX tool. There are differences in approach with some guidelines recommending fixed intervention thresholds, while others have proposed age-dependent thresholds or, in a minority of cases, a hybrid of age-dependent and fixed thresholds. In the UK, NOGG developed a guideline on the basis of clinical appropriateness, setting the threshold at the age-specific probability of fracture equivalent to a woman having already sustained a fracture. The latter process is supported by, but not dependent on, the demonstration of cost effectiveness; it avoids inappropriate over-treatment of older individuals and under-treatment of younger individuals.
Cost-effectiveness is accepted in many countries as a primary driver of decisions about who should be offered treatment with particular medications. Such approaches arose from the perceived need to limit access to expensive treatments across a range of diseases. Within osteoporosis, for example, health technology assessments of available treatments in the UK by the NICE have traditionally stratified the risk levels at which treatments could be initiated [125,126,127,128,129]. This stratification was usually based on age, BMD T score and the presence of one or more individual clinical risk factors; the cost-effectiveness thresholds were subsequently assumed by the clinical community to be intervention thresholds. More recently, NICE appraisals have embraced the use of absolute fracture risk, but the continued application of cost-effectiveness to developing thresholds for now inexpensive drugs led to counterintuitive and potentially harmful guidance [130].
In summary, many countries have access to evidence-based policies and guidelines covering which individuals should be offered treatment and the appropriate treatment to be offered.
Clinical management of the condition and patient outcomes should be optimised in all healthcare providers prior to participation in a screening programme
A general approach to reducing the fracture burden in society was outlined by the Department of Health in the UK in 2009 (Fig. 2). This approach recognized the need to start by providing optimal care to those with hip fractures, followed by the provision of services that would identify, investigate and treat those presenting with non-hip fragility fractures (so-called secondary prevention).
A standardized approach to post hip fracture care was first published in a collaboration between the British Orthopaedic Association and British Geriatric Society in 2007 [131], followed soon after by the establishment of the National Hip Fracture Database to audit hospital performance. The database is centrally funded via government but is run independently. Importantly, the programme was subsequently supported by the creation in 2010 of a Best Practice Tariff (BPT) to financially incentivize improved care. Subsequently, NICE incorporated much of this acute hip fracture management initiative into their own guideline in 2011 [132]. The guidelines state that patients should be managed with combined orthopaedic and orthogeriatric care in a hip fracture programme with all patients preferably being seen preoperatively; due to difficulties in accessing orthogeriatric services at weekends, the target is that all patients are reviewed within 72 h from admission. Notably, 30-day mortality fell by 7.6% per year in the 4 years after the introduction of the National Hip Fracture Database compared to a 1.8% per year decrease in the 4 years preceding its introduction [133]. Similar systems are being established in other European countries [134].
In the next tier of the pyramid in Fig. 2, it was recognized that patients presenting with a fragility fracture, related or unrelated to a fall, should be assessed for osteoporosis and receive effective management to improve their bone health and reduce future fracture risk. The preferred solution is the establishment of Fracture Liaison Services, which are usually hospital-based and provide a co-ordinator to identify patients aged 50 and over with a first fracture [135, 136]. The identification is followed by risk assessments and initiation of evidence-based interventions for bone health and falls prevention. Ideally, the service would also monitor adherence and any recurrent events, but this is frequently passed back to primary care services. Treatment includes prescribing bone strengthening medicines, as well as referral to falls risk assessment and prevention services where appropriate. To date, no randomised control trials have demonstrated the effectiveness of FLS in reducing fracture risk, but such trials have shown increased DXA testing, treatment initiation and early adherence in FLS-like settings [137,138,139]. Observational studies have shown reductions in fracture risk, though somewhat inconsistently, with difficulty in interpreting the results due to a number of biases in such study designs [140]. Many of these biases were overcome in a recent Swedish study, comparing fracture rates before and after FLS implementation, with non-FLS hospitals as an added comparator [141]. The risk of recurrent fracture was 18% lower in the period post FLS implementation compared with the control period (hazard ratio = 0.82, 95% confidence interval [CI] 0.73–0.92, p = 0.001), with no change in recurrent fracture rate in the non-FLS hospitals [141]. The number and standard of FLS worldwide continues to increase under the auspices of the International Osteoporosis Foundation’s Capture The Fracture ® programme (https://www.capturethefracture.org/).
There is some overlap between the next two tiers of the strategy shown in Fig. 2, namely individuals at highest risk of fragility fracture and older people, as age itself is a prominent risk factor for fracture. The individuals encompassed by these tiers are largely those where the burden of osteoporosis assessment and management falls on primary care health professionals, and it is this constituency to which the proposed screening approach would be largely directed. There is good evidence that, in this setting, treatment rates are very low. For example, in a recent study, treatment rates in women ≥ 70 years, deemed to be at high risk of fracture, were examined in primary care in 8 European countries [142]. The women were enrolled when visiting their general practitioner, regardless of the reason for attendance, and data captured on fracture risk and osteoporosis treatments. Increased risk of fracture was characterized as one or more of the following, a history of fracture, FRAX 10-year probability of fracture above country-specific thresholds, or a T score ≤ − 2.5 at the spine or hip. In the 3798 enrolled patients, median FRAX probability (calculated without BMD) was 7.2% for hip fracture and 16.6% for major osteoporotic fracture. Overall, 2077 women (55%) met one or more of the criteria for increased risk of fracture (median 10-year probabilities of hip and major osteoporotic fracture 11.2% and 22.8% respectively). An osteoporosis diagnosis was recorded in 804 patients (21%); most (80%) of these were at increased fracture risk. The treatment gap (the proportion at high risk but untreated) was 75%, varying from 53% in Ireland to 91% in Germany [142]. The treatment gap was somewhat lower in women with an osteoporosis diagnosis compared to those without (31% vs. 94% respectively). These data are very similar to osteoporosis treatment gaps assessed in other recent studies [11, 143], and reflect the need for integrated approaches to fracture prevention [144].
As described above, strides are being made in the clinical management of fracture risk, but the impact on patient and healthcare outcomes remains sub-optimal. The proposed screening program, if well implemented, would actually address the current deficiencies in care of a high-risk subgroup within the population, i.e. it should be regarded as a means to optimize healthcare in a greater proportion of those at risk.
The screening programme
There should be evidence from high quality randomised controlled trials that the screening programme is effective in reducing mortality or morbidity. Where screening is aimed solely at providing information to allow the person being screened to make an “informed choice”, there must be evidence from high quality trials that the test accurately measures risk. The information that is provided about the test and its outcome must be of value and readily understood by the individual being screened.
As stated earlier, the proposed screening programme is based on the randomised, controlled SCOOP study which is described in more detail below [3]. Two additional randomised studies, namely the Risk-stratified Osteoporosis Strategy Evaluation study (ROSE) from Denmark [145] and the SALT Osteoporosis Study (SOS) from the Netherlands [146], have used FRAX-based approaches for population screening and are also discussed below.
The SCOOP study
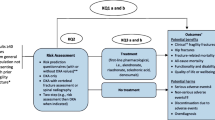
The Screening for Osteoporosis in Older People (SCOOP) study was a multicentre, primary care-based screening programme [3]. Approximately 52,000 women age 70–85 years were identified in 100 primary care practices in England; when those with exclusion criteria which included ongoing treatment for osteoporosis and certain concurrent conditions (e.g. known dementia, terminal illness or recent bereavement), letters of invitation were sent to 38,600 women. Of the latter, 25,571 women did not respond (n = 11,068) or declined the invitation (n = 13,870), leaving 13,029 eligible participants. A total of 12,483 (95.8% of eligible, 32.3% of those invited) women were finally included in the randomised controlled trial which had a planned follow-up of 5 years.
The steps in the screening protocol are outlined in Table 4. It should be noted that the FRAX risk assessment was on the basis of 10-year hip fracture probability. It was undertaken at study enrolment in the screening arm and, in the control arm, calculated using the baseline questionnaire at the end of the study. In the latter, fracture risk assessment and management were left to normal clinical practice under the care of the GP. In the screening arm, hip fracture probabilities were calculated from the self-completed risk questionnaires; if the 10-year probability was above an age-dependent assessment threshold, the participant was invited for a DXA scan to measure femoral neck BMD. Those with probabilities below the threshold were informed that they were at low risk of hip fracture. In those attending for a DXA scan, the femoral neck BMD was then used in the recalculation of their 10-year hip fracture probability. If the latter value lay at or above an age-dependent intervention threshold, the participant and their GP were informed of a high risk of hip fracture and treatment, largely with oral bisphosphonates, was recommended.
In the screening arm, only 898 women (14.4% of the group) were identified as at high risk of hip fracture and recommended for treatment. Overall, the screening strategy did not reduce the incidence of all osteoporosis related fracture, (hazard ratio [HR] 0.94, 95% CI 0.85–1.03, p = 0.178), but the number of hip fractures in the screening arm was significantly lower than that in the control arm (164 vs. 218 respectively, HR 0.72, 0.59–0.89, p = 0.002) [3]. The lack of DXA scanning in the control arm restricted any subsequent comparisons between the two study arms to FRAX probabilities calculated without BMD. A subsequent analysis showed a significant interaction between baseline FRAX hip fracture probability and the effectiveness of screening to reduce the incidence of hip fractures, i.e. the reduction was only seen at high baseline risks associated with the recommendation for treatment [4]. Another approach to determine the contribution of intervention in such a small proportion of the screening arm to the overall hip fracture reduction is shown in Fig. 3. Here, a comparison of observed versus expected number of hip fractures was undertaken; the expected number of hip fractures was calculated from baseline FRAX probabilities using an adaptation of the FRAX tool with a 5-year time horizon to reflect the duration of the SCOOP study.
The adapted FRAX tool predicted that a total of 212 women (3.39%) in the control arm would sustain one or more hip fractures during the SCOOP study; the actual number with incident hip fractures was 218 (3.49%), an observed to expected ratio (O/E ratio) of 1.03 (95%CI 0.90–1.18). In the screening arm, in contrast to an expected number of 212 women with incident hip fractures, only 164 women (2.63%) sustained such fractures, with an O/E ratio of 0.77 (95%CI 0.66–0.90). In the 898 women allocated to the high risk group in the screening arm and recommended for treatment, a total of 70 hip fractures were expected, but only 39 were observed (O/E ratio 0.56, 0.40–0.77). Thus, of the 48 fewer hip fractures than expected, 31 (64.6%) were in this small subgroup (14.4% of the screening group).
The numbers needed to screen (NNS) to prevent one osteoporotic or hip fracture in SCOOP were 133 and 115 respectively; for the same outcomes, the numbers needed to treat (NNT) were 19 and 17 respectively (Table 4).
The ROSE study
The ROSE study in southern Denmark was a randomised controlled trial in 34,229 women aged 65–80 years, to investigate the effectiveness of a two-step population-based osteoporosis screening programme using FRAX® to select women for DXA scans [145]. The steps in the screening protocol are outlined in Table 4.
Randomisation to screening (n = 17,072) or control (n = 17,157) groups took place before the letters of invitation were sent out. Those invited were asked to return a self-completed questionnaire, including the FRAX risk factors. A total of 27,157 women (79.3% of the cohort) returned the questionnaires, but FRAX probabilities were only calculable in 20,905 women (61% of cohort). Following the exclusion of women already on osteoporosis treatment, 18,605 women (54.4%) were included in the next stage of the study where the FRAX 10-year major osteoporotic fracture probability was used to determine the need for a DXA scan to measure BMD (termed the per protocol analysis 1 population). If the FRAX MOF probability was 15% or greater, the women were recommended a DXA scan; this comprised 7056 (76%) out of 9279 women in the screening arm of the study, but DXA scans were only obtained in 5009 women (54% of the high-risk subgroup). Following the DXA scan, osteoporosis treatment was indicated based on criteria defined within Danish guidelines; in brief, treatment was recommended via the GP or specialist clinic if the BMD T score at the spine or total hip was ≤ − 2.5, if a vertebral fracture was detected on lateral spine imaging during the DXA assessment, or if the BMD T score was < − 1.0 and the woman was receiving ongoing supraphysiologic doses of glucocorticoids [147]. In the screening arm, treatment was recommended in 1236 women and was started in 986 women. Thus, in the screening arm, treatment was recommended in 7.2% of all the women randomised and invited to take part, or 13.3% if the denominator is the number of women completing a FRAX assessment (per protocol analysis 1).
Overall, in 10, there was no difference in incident fractures (MOF, hip fracture, and all fractures) between the screening and control arms during a follow-up of approximately 5 years. In contrast, in the per-protocol analysis 1, while no difference was observed in all incident fractures or MOF, a borderline significant reduction in hip fracture risk was observed in the screened group (169 vs 202 hip fractures, HR 0.82, 95%CI 0.67–1.01, p = 0.059). The effect was even more marked in the smaller subgroup who attended for DXA scans (HR 0.74, 95%CI 0.58–0.95, p = 0.018), but the latter needs to be interpreted with caution due to the relatively large dropout rate (46%) in those recommended for DXA scans [145].
The NNS to prevent one osteoporotic or hip fracture in ROSE were 319 and 281 respectively; for the same outcomes, the NNT were 34 and 30 respectively (Table 4).
The SOS study
SOS in the Netherlands was also a pragmatic randomised controlled trial in women aged 65–90 years with at least 1 clinical risk factor for fracture [146]. The latter included previous fracture after age 50 years, parental hip fracture, BMI < 19 kg/m2, rheumatoid arthritis, early menopause (< 45 years of age), malabsorption syndrome, chronic liver disease, type I diabetes mellitus or immobility (severe walking difficulties and/or use of walking aid). Exclusion criteria included a predicted short life expectancy, current or recent osteoporosis treatment, high body weight (> 135 kg) or glucocorticoid use ≥ 7.5 mg prednisone equivalent/day.
From a total pool of almost 54,000 women in primary care, 25,314 completed a baseline questionnaire and 11,032 fulfilling the inclusion/exclusion criteria were entered into the study. Of the 5575 women randomised to the screening arm, the screening procedures were only conducted in 4228 (75.8%). The procedures included DXA, vertebral fracture assessment (VFA), FRAX assessment, falls history and blood tests to exclude secondary osteoporosis. Following this, treatment was recommended in 1417 women (25% of all women randomised to screening or 33.5% of those undergoing the screening procedures). Treatment criteria predominantly comprised a FRAX 10-year MOF probability, calculated with BMD, greater than age-dependent thresholds (these ranged from > 15% in women aged 60–65 years, to > 32% in women aged 85–91 years). This criterion did not mandate treatment if the T score was > − 2.0; 170 women were excluded from treatment on this basis [148]. Some women also received treatment if they fulfilled the criteria published within Dutch guidelines but did not reach the FRAX-based thresholds; an additional 36 women were treated on this basis [148].
Over a planned study follow up of 3 years, 626 women in the screening group had a fracture compared to 632 in the usual care group (HR 0.97, 95%CI 0.87–1.08). The point estimate of the hazard ratio for other fracture outcomes, including osteoporotic fractures, major osteoporotic fractures and hip fractures was somewhat lower (0.91), but did not reach statistical significance. For example, 133 women sustained hip fractures in the screening arm compared to 143 women in the usual care arm (HR 0.91, 95% CI 0.71–1.15). In an exploratory analysis among participants with a recent fracture (< 2 years before baseline), fewer women sustained hip fractures in the screening arm but the number of events was small (10 versus 25, HR 0.38; 95% CI 0.18–0.79). The authors noted that the outcome of the trial may have been impacted by nonparticipation and medication nonadherence in the screening group.
The NNS to prevent one osteoporotic or hip fracture in SOS were 178 and 552 respectively; for the same outcomes, the NNT were 32 and 98 respectively (Table 4).
Meta-analysis of FRAX-based screening RCTs
As part of a systematic review of randomised studies of screening for high fracture risk, the investigators of the SOS study subsequently undertook a meta-analysis of the three studies described in detail above [149]. This analysis comprised a total of 42,009 participants, reflecting all the women participating in the SCOOP and SOS studies, plus all the women providing a FRAX-based assessment at entry to the ROSE study. The latter group was deemed to be comparable to the participants in the other two studies. The proportions receiving treatment recommendations in the studies were 13% in ROSE, 14% in SCOOP and 25% in SOS, with subsequently 11%, 15%, and 18% respectively commencing therapy. Despite these relatively low treatment rates, the meta-analysis showed a statistically significant decrease of 5% in the incidence of osteoporotic fractures (HR 0.95, 95% confidence interval (CI) 0.89–1.00) when comparing screening to usual care. Most notably, there was a 20% decrease in hip fractures (HR 0.80; 95%CI 0.71–0.91) (Fig. 4A). There was also a 9% decrease in major osteoporotic fractures (HR = 0.91; 0.84–0.98), but this analysis didn’t include the outcome of major osteoporotic fractures in 9 (HR 0.88, 0.79–0.98, p = 0.018) [150]. Addition of the SCOOP result to the meta-analysis resulted in a similar overall reduction in major osteoporotic fractures (Fig. 4B). There was no difference in all-cause mortality (HR 1.04; 0.95–1.14). It is worth noting that both ROSE and SOS used the FRAX MOF output to target assessment; if the FRAX Hip output had been used as in 9, it is possible that the reduction in hip fractures would have been somewhat greater.
Forest plots of screening for prevention of hip (A, adapted from [149]) and major osteoporotic (B) fractures versus usual care. Note: From the ROSE study, the data from the first per protocol analysis were used, as these were most comparable to the data from the SCOOP and SOS studies. In B, the meta-analysis from [149] has been updated to include the major osteoporotic fracture outcome from the SCOOP study. A Outcome — hip fracture, B Outcome — major osteoporotic fracture
In the pooled cohort, the NNS and NNT for hip fractures were 272 and 28 respectively. The meta-analysis clearly showed population screening to be effective, with the biggest reduction observed in the outcome of hip fracture, leading the authors to conclude that implementation of screening in older women should be considered a serious option.
In summary, three randomised controlled studies and a subsequent meta-analysis have established efficacy for the use of FRAX screening to reduce the risk of hip fracture, as well as other fracture outcomes.
There should be evidence that the complete screening programme (test, diagnostic procedures, treatment/ intervention) is clinically, socially and ethically acceptable to health professionals and the public.
None of the steps or assessments in the proposed screening programme is novel. The FRAX questionnaire has been used in routine clinical practice since its launch in 2008. The website alone, which is not the only means of accessing the FRAX calculation, has had approximately 34 million calculations over the last 10 years (site accessed on 16th July 2021). In a recent review, there was widespread usage of the online tool globally [98], with its daily place in the management of patients at risk of fracture reflected in the marked downturn in usage associated with the onset of the global pandemic [151].
Bone mineral density measurement by DXA has been part of routine clinical care of osteoporosis since the 1980s and is a widely accepted technique for both patients and doctors. The radiation dose is low (equivalent to 2–3 days of background radiation) [152], the scanning time is rapid (a few minutes) and the scan does not require the patient to be enclosed or in a tunnel.
A randomized controlled trial of screening using BMD found no evidence of an adverse effect on either quality of life or anxiety [153], while qualitative studies have reported some anxiety (i.e. a low BMD conveying a feeling of weakness) but others have reported more positive views of BMD measurement [154, 155]. A specific assessment of the acceptability of the proposed screening approach used in the SCOOP study has been published [99]. The results showed that women and GPs viewed screening positively, recognizing its potential to improve fracture prevention and future health.
In summary, none of the components of the screening programme are new or untested, with many years of experience suggesting that the programme is clinically, socially and ethically acceptable to health professionals and the public.
The benefit gained by individuals from the screening programme should outweigh any harms, for example from overdiagnosis, overtreatment, false positives, false reassurance, uncertain findings and complications.
As noted, qualitative data indicate that the proposed screening programme is not associated with any increase in anxiety. The numbers needed to treat to prevent one hip fracture, derived from the meta-analysis, compare very favourably with those for the use of statins to prevent non-fatal myocardial infarctions or strokes in patients with (https://www.thennt.com/nnt/statins-for-heart-disease-prevention-with-known-heart-disease/) or without (https://www.thennt.com/nnt/statins-for-heart-disease-prevention-without-prior-heart-disease-2/) known heart disease. The screening test identifies a risk or, more accurately in the case of FRAX, a probability, rather than a specific disease or a pre-disease state; the use of false positives and false negatives in this setting is complex. The positive and negative predictive values are probably of most importance to clinicians and patients alike, combined with information on the safety and efficacy of the treatments. There is therefore a need to ensure that the individual patient’s understanding of risk is addressed, that their understanding of the importance of the adverse outcome (i.e. fracture, particularly hip fracture) is good, and the benefits and risks of the treatment or intervention are clear. All the treatments used in 8 are licensed and many are long-established with very well characterised data on efficacy and side effects. Public and individual awareness of rare side effects of osteoporosis treatments, namely atypical femoral fractures (AFF) and osteonecrosis of the jaw (ONJ), can impact on treatment uptake despite occurring at very low rates in the treated osteoporosis population (approximately 1 in 1000–100,000 for AFFs and 1 in 10,000–100,000 for ONJ) and usually only after several years of exposure [156,157,158,159]. The risks of these outcomes are far outweighed by the number of fractures at the hip and other sites prevented by treatment [156, 160].
The opportunity cost of the screening programme (including testing, diagnosis and treatment, administration, training and quality assurance) should be economically balanced in relation to expenditure on medical care as a whole (value for money). Assessment against these criteria should have regard to evidence from cost benefit and/or cost effectiveness analyses and have regard to the effective use of available resource.
Several cost-effectiveness analyses of population screening strategies in osteoporosis have been published over the last 20 years, but relatively few have been tested in randomized controlled trials [153, 161]. Of the three recent randomised controlled studies using FRAX, only the SCOOP study has published cost-effectiveness analyses to date. The first of these used a within-trial design limited to the time frame of the trial (5 years), with questionnaires (EQ-5D-3L) administered to document self-reported quality of life (QoL) at 6-month intervals during the first year and then at annual intervals used to derive QALYs[6]. The screening arm had a non-significant average incremental QALY gain of 0.0237 (95% CI –0.0034 to 0.0508) for the 5-year follow-up, with an incremental cost per QALY gained of £2772 compared with the control arm. Cost-effectiveness acceptability curves indicated a 93% probability of the intervention being cost-effective at values of a QALY greater than £20,000. The authors concluded that the analysis demonstrated that a systematic, community-based screening programme of fracture risk in older women in the UK represents a highly cost-effective intervention. The most recent analysis used a more established health economic Markov model study design, whereby disutilities of fractures, derived from other quality of life studies, were applied to the women sustaining incident fractures [162]. In addition, the cost-effectiveness analysis modelled a linear offset of effect of the screening programme and calculated the cost-effectiveness over a longer time horizon (remaining lifetime). The model was populated with costs related to drugs, administration and screening intervention derived from the SCOOP study with fracture risk in the control and screening arms corresponding to the observed risk observed in the study. The analysis reported that screening of 1000 patients would save 9 hip fractures and 20 non-hip fractures over the remaining lifetime (mean 14 years) and save costs (£286) in comparison with usual management. The analysis strongly suggests that there are no opportunity costs to a screening programme based on the SCOOP study, and its implementation could actually be cost saving.
Thus, the opportunity costs of the screening programme are low or even cost-saving. They are comparable to or better than many public health measures [163] or other established screening programmes [164, 165]
All other options for managing the condition should have been considered (such as improving treatment or providing other services), to ensure that no more cost effective intervention could be introduced or current interventions increased within the resources available.
Current approaches to fracture prevention are addressed in the section on clinical management of the condition above. In the Department of Health guidance in the UK, a focus is given to interventions that will reduce falls risk and/or fracture risk [166]. Falls reduction strategies are also recommended in many international guidelines as a means of reducing falls-related injuries, of which fracture is one of the most serious consequences, especially hip fracture. While assessment of falls risk and appropriate interventions aimed at reducing falls risk has been shown to be effective, at least in the short term, their impact on the risk of hip fracture is less certain [104, 107]. Furthermore, the reduction in hip fractures in the SCOOP study was not mediated by any impact of the screening programme on falls risk [167].
In summary, while falls prevention measures should continue to be included in individual patient management, the role for bone-targeted treatments should be prioritised.
Conclusion
In this paper, we have sought to assess the potential for a screening programme to identify women at increased risk of hip fracture. We have approached this by proposing a programme based on that used successfully in the SCOOP study and then addressing 15 of the 19 criteria established by the UK National Steering Committee for the assessment of such a programme (Table 5). Notably, evidence addressing these criteria were reviewed for osteoporosis screening by the UK NSC in 2019 (evidence cut-off September 2018), following an external review, and concluded screening could not currently be recommended [168, 169]. A similar conclusion was reached in an assessment under an EU Health Technology Assessment report also published in 2019 [170]. These conclusions were largely based on areas of continuing uncertainty which may be seen as relevant to screening in the UK and beyond. For example, uncertainty remained about the accuracy of screening tests in women who would be included in a population screening programme. We would argue that there is compelling evidence from systematic reviews and individual studies that the accuracy for future hip fractures, as reflected by the AUCs, is appropriate for a clinical tool for the prediction of future events as opposed to the detection of existing or early disease as in other screening programmes [171].
Uncertainty has also been expressed about the effect of treatment and changes in lifestyle on some types of fracture, and on fracture risk in women identified as being at risk of fracture through screening. It is widely recognised that the effect of anti-osteoporosis treatments differs across skeletal sites. For example, analyses consistently show the largest reductions in vertebral fractures (50–70%), intermediate reductions in hip fractures (40–50%) and somewhat lower reductions in non-vertebral, non-hip fractures (20–25%). Both the UK NSC and the EU HTA reports acknowledged that there was evidence of potential benefit for hip fractures [168,169,170], though neither conducted a meta-analysis that combined the similar populations in both the SCOOP and ROSE studies (i.e. confined to those who returned a baseline risk questionnaire). It is also important to bear in mind that unlike randomised controlled trials of treatments versus placebo, the recent studies of screening have examined strategies rather than treatment; importantly, the proportion targeted for treatment in these studies was small (e.g. 14% in SCOOP) meaning that the majority of participants in both arms of the studies did not receive any therapeutic intervention. A 25% reduction in non-vertebral, non-hip fractures during treatment would be manifest by only a 3.5% reduction in the whole cohort where treatment was just targeted to 14% of the population. It should also be noted that the proposed screening program, predicated on FRAX 10-year probability of hip fracture, targets interventions to individuals with a higher risk of a fracture that is more reversible by osteoporosis treatments. A previous post hoc analysis within the SCOOP study showed that the reductions in hip fractures at baseline high FRAX hip probability are of a similar magnitude to that observed in randomised controlled studies of antiresorptive treatments, an observation that is supported by the analysis presented in this report of the 55% reduction in hip fractures in the small high-risk cohort. We would conclude that there is good evidence that women identified by screening are responsive to appropriate management with anti-osteoporosis agents, particularly with regard to prevention of hip fractures as reflected in the recent meta-analysis.
Finally, uncertainties have been raised about how much added benefit would be gained by population screening over usual care, and the cost-effectiveness of a population screening programme. While none of the screening studies were designed to directly capture impacts on morbidity and mortality, hip fractures are well documented to result in significant morbidity, hospitalisation, surgery and prolonged rehabilitation with a significant number of patients not returning to their pre-fracture level of mobility and independence. The proposed strategy combines a low-cost assessment with targeted intervention using low-cost generic treatments, predominantly oral bisphosphonates; the subsequent 28% reduction in the incidence of hip fractures is a highly impactful strategy compared to usual care. Even more importantly, the standard cost-effectiveness analysis demonstrates that the strategy dominates usual care, i.e. it is actually cost saving. Future health economic modelling should enable assessment of different screening strategies for hip fracture risk, including the wider utilisation of BMD as part of the strategy.
The major limitation of this report, perhaps, is that it is not based on a systematic review to address the available literature on the areas addressed by each specific criterion, and the selected randomised controlled trials excluded other non-FRAX approaches. For example, we did not include a study that addressed screening for prevalent vertebral fractures as the latter was of relatively short duration (1 year) and had medication uptake as the main endpoint [172]. However, the expert panel comprises a wide, in-depth knowledge and expertise in the fields of risk assessment, guidelines, treatment and health economics of osteoporosis. The approach used attempted to measure the level of consensus on the various criteria from a wide variety of geographic and healthcare system settings. The report is meant to serve as a basis for engagement and discussion with policy makers and payers around the potential for screening in osteoporosis with a particular focus on high hip fracture risk.
From the evidence reviewed in this report, we would contend that the case for screening to identify women at increased risk of hip fracture is now made and that future research should focus on strategies for optimal implementation of this approach across different healthcare systems and settings. We would certainly conclude that any transition towards screening that leads to improved identification of hip fracture risk in older women in primary care (e.g. enhanced case-finding) will undoubtedly have positive impacts on the clinical, personal and economic burden of this most serious of osteoporotic fractures.
References
Wilson JMG, Jungner G (1968) Principles and practice of screening for disease. World Health Organization https://apps.who.int/iris/handle/10665/37650. Accessed 18 Aug 2021
Shepstone L, Fordham R, Lenaghan E et al (2012) A pragmatic randomised controlled trial of the effectiveness and cost-effectiveness of screening older women for the prevention of fractures: rationale, design and methods for the SCOOP study. Osteoporos Int 23:2507–2515
Shepstone L, Lenaghan E, Cooper C et al (2018) Screening in the community to reduce fractures in older women (SCOOP): a randomised controlled trial. Lancet 391:741–747
McCloskey E, Johansson H, Harvey NC et al (2018) Management of patients with high baseline hip fracture risk by FRAX reduces hip fractures-a post hoc analysis of the SCOOP study. J Bone Miner Res 33:1020–1026
Parsons CM, Harvey N, Shepstone L et al (2019) Systematic screening using FRAX((R)) leads to increased use of, and adherence to, anti-osteoporosis medications: an analysis of the UK SCOOP trial. Osteoporos Int 31:67–75
Turner DA, Khioe RFS, Shepstone L et al (2018) The cost-effectiveness of screening in the community to reduce osteoporotic fractures in older women in the UK: economic evaluation of the SCOOP study. J Bone Miner Res 33:845–851
Kanis JA, Oden A, McCloskey EV, Johansson H, Wahl DA, Cooper C (2012) A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int 23:2239–2256
Anon (1993) Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 94:646–650
Leslie WD, Morin S (2011) Fracture burden in relation to low bone mineral density and FRAX((R)) probability. J Clin Densitom 14:279–285
Svedbom A, Ivergard M, Hernlund E, Rizzoli R, Kanis JA (2014) Epidemiology and economic burden of osteoporosis in Switzerland. Arch Osteoporos 9:187
Kanis JA, Norton N, Harvey NC, Jacobson T, Johansson H, Lorentzon M, McCloskey EV, Willers C, Borgstrom F (2021) SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch Osteoporos 16:82
Borgstrom F, Karlsson L, Ortsater G et al (2020) Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos 15:59
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733
Cooper C, Cole ZA, Holroyd CR, Earl SC, Harvey NC, Dennison EM, Melton LJ, Cummings SR, Kanis JA, Epidemiology ICWGoF (2011) Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int 22:1277–1288
Lorentzon M, Johansson H, Harvey NC, Liu E, Vandenput L, McCloskey EV, Kanis JA (2022) Osteoporosis and fractures in women: the burden of disease. Climacteric 25:4–10
Svedbom A, Hernlund E, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA (2013) Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos 8:137
Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8:136
Oden A, McCloskey EV, Kanis JA, Harvey NC, Johansson H (2015) Burden of high fracture probability worldwide: secular increases 2010–2040. Osteoporos Int 26:2243–2248
Kanis JA, Oden A, McCloskey EV, Johansson H, Wahl DA, Cooper C, Epidemiology IOFWGo, Quality of L, (2012) A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int 23:2239–2256
Berry SD, Miller RR (2008) Falls: epidemiology, pathophysiology, and relationship to fracture. Curr Osteoporos Rep 6:149–154
Gibson M (1987) The prevention of falls in later life. Dan Med Bull 34(Suppl 4):1–24
Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A (2001) The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 12:417–427
Keene GS, Parker MJ, Pryor GA (1993) Mortality and morbidity after hip fractures. BMJ 307:1248–1250
Poor G, Atkinson EJ, O’Fallon WM, Melton LJ 3rd (1995) Determinants of reduced survival following hip fractures in men. Clin Orthop 319:260–265
Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK (2003) The components of excess mortality after hip fracture. Bone 32:468–473
Johnell O, Kanis JA, Oden A et al (2005) Predictive value of BMD for hip and other fractures. J Bone Miner Res 20:1185–1194
WHO (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organization, Geneva
Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9:1137–1141
Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ 3rd, Khaltaev N (2008) A reference standard for the description of osteoporosis. Bone 42:467–475
US Preventive Services Task Force (2011) Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med 154:356–364
US Preventive Services Task Force (2018) Screening for osteoporosis to prevent fractures US preventive services task force recommendation statement. JAMA 319(24):2521–2531. https://doi.org/10.1001/jama20187498
Collins GS, Altman DG (2010) An independent and external validation of QRISK2 cardiovascular disease risk score: a prospective open cohort study. BMJ 340:c2442
D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB (2008) General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 117:743–753
Goff DC Jr, Lloyd-Jones DM, Bennett G et al (2014) 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 63:2935–2959
De Laet C, Kanis JA, Oden A et al (2005) Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int 16:1330–1338
Kanis JA, Johnell O, De Laet C et al (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35:375–382
Kanis JA, Johansson H, Oden A et al (2004) A family history of fracture and fracture risk: a meta-analysis. Bone 35:1029–1037
Kanis JA, Johansson H, Oden A et al (2004) A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res 19:893–899
Xue AL, Wu SY, Jiang L, Feng AM, Guo HF, Zhao P (2017) Bone fracture risk in patients with rheumatoid arthritis: a meta-analysis. Medicine (Baltimore) 96:e6983
Vilaca T, Walsh J, Eastell R (2019) Discordant pattern of peripheral fractures in diabetes: a meta-analysis on the risk of wrist and ankle fractures. Osteoporos Int 30:135–143
Janghorbani M, Van Dam RM, Willett WC, Hu FB (2007) Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 166:495–505
Leslie WD, Rubin MR, Schwartz AV, Kanis JA (2012) Type 2 diabetes and bone. J Bone Miner Res 27:2231–2237
Kanis JA on behalf of the World Health Organization Scientific Group (2007) Assessment of osteoporosis at the primary health-care level. Technical Report. World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK, University of Sheffield
Feskanich D, Willett W, Colditz G (2002) Walking and leisure-time activity and risk of hip fracture in postmenopausal women. JAMA 288:2300–2306
Leslie WD, Morin SN, Lix LM, Martineau P, Bryanton M, McCloskey EV, Johansson H, Harvey NC, Kanis JA (2019) Fracture prediction from self-reported falls in routine clinical practice: a registry-based cohort study. Osteoporos Int 30:2195–2203
Morrison A, Fan T, Sen SS, Weisenfluh L (2013) Epidemiology of falls and osteoporotic fractures: a systematic review. Clinicoecon Outcomes Res 5:9–18
Kanis JA, Johnell O, Oden A et al (2005) Smoking and fracture risk: a meta-analysis. Osteoporos Int 16:155–162
Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, Eisman JA, Pols H, Tenenhouse A (2005) Alcohol intake as a risk factor for fracture. Osteoporos Int 16:737–742
Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D (2019) Pharmacological management of osteoporosis in postmenopausal women: an endocrine society* Clinical Practice Guideline. J Clin Endocrinol Metab 104:1595–1622
Compston J, Cooper A, Cooper C et al (2017) UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 12:43
Kanis JA, Cooper C, Rizzoli R, Reginster JY, Scientific Advisory Board of the European Society for C, Economic Aspects of O, the Committees of Scientific A, National Societies of the International Osteoporosis F (2019) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 30:3–44
Papaioannou A, Morin S, Cheung AM et al (2010) 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 182:1864–1873
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R (2014) Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int 25:2359–2381
Harvey NC, Biver E, Kaufman JM et al (2017) The role of calcium supplementation in healthy musculoskeletal ageing : an expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF). Osteoporos Int 28:447–462
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine S (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930
Scientific Advisory Committee on Nutrition (2016) Vitamin D and health https://www.gov.uk/government/groups/scientific-advisory-committee-on-nutrition. Accessed 18 Aug 2021
Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, Stepan J, El-Hajj Fuleihan G, Bouillon R (2019) Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol 180:P23–P54
Society NO (2013) Vitamin D and bone health: a practical clinical guideline for patient management. https://www.nos.org.uk/document.doc?id=1352. Accessed 18 Aug 2021
Weaver CM, Alexander DD, Boushey CJ, Dawson-Hughes B, Lappe JM, LeBoff MS, Liu S, Looker AC, Wallace TC, Wang DD (2016) Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int 27:367–376
Weaver CM, Dawson-Hughes B, Lappe JM, Wallace TC (2016) Erratum and additional analyses re: Calcium plus vitamin D supplementation and the risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int 27:2643–2646
Zhao JG, Zeng XT, Wang J, Liu L (2017) Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA 318:2466–2482
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19:385–397
Kanis J.A. on behalf of the WHO Scientific Group (2008) Assessment of osteoporosis at the primary health-care level. Technical Report. WHO Collaborating Centre, University of Sheffield, UK, Sheffield
Hippisley-Cox J, Coupland C (2012) Derivation and validation of updated QFracture algorithm to predict risk of osteoporotic fracture in primary care in the United Kingdom: prospective open cohort study. BMJ 344:e3427
Hippisley-Cox J, Coupland C (2009) Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores. BMJ 339:b4229
Nguyen ND, Eisman JA, Center JR, Nguyen TV (2007) Risk factors for fracture in nonosteoporotic men and women. J Clin Endocrinol Metab 92:955–962
Kanis JA, Harvey NC, Cooper C, Johansson H, Oden A, McCloskey EV, Advisory Board of the National Osteoporosis Guideline G (2016) A systematic review of intervention thresholds based on FRAX: a report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos 11:25
Johansson H, Kanis JA, Oden A, Compston J, McCloskey E (2012) A comparison of case-finding strategies in the UK for the management of hip fractures. Osteoporos Int 23:907–915
McCloskey E, Kanis JA, Johansson H et al (2015) FRAX-based assessment and intervention thresholds–an exploration of thresholds in women aged 50 years and older in the UK. Osteoporos Int 26:2091–2099
Kanis JA, Johansson H, Harvey NC, McCloskey EV (2018) A brief history of FRAX. Arch Osteoporos 13:118
Kanis JA, Harvey NC, Johansson H, Liu E, Vandenput L, Lorentzon M, Leslie WD, McCloskey EV (2020) A decade of FRAX: how has it changed the management of osteoporosis? Aging Clin Exp Res 32:187–196
Kanis JA, Johnell O (2005) Requirements for DXA for the management of osteoporosis in Europe. Osteoporos Int 16:229–238
Clynes MA, Westbury LD, Dennison EM et al (2020) Bone densitometry worldwide: a global survey by the ISCD and IOF. Osteoporos Int 31:1779–1786
Curtis EM, Woolford S, Holmes C, Cooper C, Harvey NC (2020) General and specific considerations as to why osteoporosis-related care is often suboptimal. Curr Osteoporos Rep 18:38–46
Harvey NC, McCloskey EV, Mitchell PJ, Dawson-Hughes B, Pierroz DD, Reginster JY, Rizzoli R, Cooper C, Kanis JA (2017) Mind the (treatment) gap: a global perspective on current and future strategies for prevention of fragility fractures. Osteoporos Int 28:1507–1529
National Institute for Health and Care Excellence (2012) NICE Clinical Guideline 146. Osteoporosis: assessing the risk of fragility fracture. https://www.nice.org.uk/guidance/cg146. Accessed 19 May 2022
Kanis JA, Oden A, Johnell O et al (2007) The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int 18:1033–1046
Chlebowski RT, Anderson GL, Lane DS, Aragaki AK, Rohan T, Yasmeen S, Sarto G, Rosenberg CA, Hubbell FA, Women’s Health Initiative I (2007) Predicting risk of breast cancer in postmenopausal women by hormone receptor status. J Natl Cancer Inst 99:1695–1705
Viswanathan M, Reddy S, Berkman N, Cullen K, Middleton JC, Nicholson WK, Kahwati LC (2018) Screening to prevent osteoporotic fractures: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 319:2532–2551
Leslie WD, Lix LM (2014) Comparison between various fracture risk assessment tools. Osteoporos Int 25:1–21
Marques A, Ferreira RJ, Santos E, Loza E, Carmona L, da Silva JA (2015) The accuracy of osteoporotic fracture risk prediction tools: a systematic review and meta-analysis. Ann Rheum Dis 74:1958–1967
Nayak S, Edwards DL, Saleh AA, Greenspan SL (2015) Systematic review and meta-analysis of the performance of clinical risk assessment instruments for screening for osteoporosis or low bone density. Osteoporos Int 26:1543–1554
Kanis JA, Oden A, Johansson H, McCloskey E (2012) Pitfalls in the external validation of FRAX. Osteoporos Int 23:423–431
Dagan N, Cohen-Stavi C, Leventer-Roberts M, Balicer RD (2017) External validation and comparison of three prediction tools for risk of osteoporotic fractures using data from population based electronic health records: retrospective cohort study. BMJ 356:i6755
Baleanu F, Iconaru L, Charles A, et al. (2021) Independent external validation of FRAX and Garvan fracture risk calculators: a sub-study of the FRISBEE cohort. Journal of Bone and Mineral Research (eprint ahead of publication) 101002/jbm410532
National Institute for Clinical Excellence (2017) Bisphosphonates for treating osteoporosis. https://www.niceorguk/guidance/ta464/resources/bisphosphonates-for-treating-osteoporosis-pdf-82604905556677. Accessed 18 Aug 2021
Harvey NC, McCloskey E, Kanis JA, Compston J, Cooper C (2017) Bisphosphonates in osteoporosis: NICE and easy? Lancet 390:2243–2244
NICE. (2017) Osteoporosis. NICE Quality Standard 149. NICE, 2017. Available at: www.nice.org.uk/qs149. Accessed 18 Aug 2021
Compston J, Bowring C, Cooper A et al (2013) Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013. Maturitas 75:392–396
Compston J, Cooper A, Cooper C, Francis R, Kanis JA, Marsh D, McCloskey EV, Reid DM, Selby P, Wilkins M (2009) Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas 62:105–108
Lekamwasam S, Adachi JD, Agnusdei D et al (2012) A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int 23:2257–2276
Clark P, Denova-Gutierrez E, Zerbini C et al (2018) FRAX-based intervention and assessment thresholds in seven Latin American countries. Osteoporos Int 29:707–715
Lesnyak O, Zakroyeva A, Babalyan V et al (2021) FRAX-based intervention thresholds in eight Eurasian countries: Armenia, Belarus, Georgia, Kazakhstan, the Kyrgyz Republic, Moldova, the Russian Federation, and Uzbekistan. Arch Osteoporos 16:87
Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M (2007) Glucocorticoid-induced osteoporosis: a systematic review and cost-utility analysis. Health Technol Assess 11:1–256
Sekhon M, Cartwright M, Francis JJ (2017) Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Serv Res 17:88
Damilakis J, Adams JE, Guglielmi G, Link TM (2010) Radiation exposure in X-ray-based imaging techniques used in osteoporosis. Eur Radiol 20:2707–2714
McCloskey EV, Johansson H, Harvey NC, Compston J, Kanis JA (2017) Access to fracture risk assessment by FRAX and linked National Osteoporosis Guideline Group (NOGG) guidance in the UK-an analysis of anonymous website activity. Osteoporos Int 28:71–76
Chotiyarnwong P, Harvey NC, Johansson H, Liu E, Lorentzen M, Kanis JA, McCloskey EV (2019) Temporal changes in access to FRAX(R) in Thailand between 2010 and 2018. Arch Osteoporos 14:66
Emmett CL, Redmond NM, Peters TJ, Clarke S, Shepstone L, Lenaghan E, Shaw AR (2012) Acceptability of screening to prevent osteoporotic fractures: a qualitative study with older women. Fam Pract 29:235–242
Kanis JA, Borgstrom F, Compston J, Dreinhofer K, Nolte E, Jonsson L, Lems WF, McCloskey EV, Rizzoli R, Stenmark J (2013) SCOPE: a scorecard for osteoporosis in Europe. Arch Osteoporos 8:144
Coronado-Zarco R, Olascoaga-Gomez de Leon A, Garcia-Lara A, Quinzanos-Fresnedo J, Nava-Bringas TI, Macias-Hernandez SI (2019) Nonpharmacological interventions for osteoporosis treatment: systematic review of clinical practice guidelines. Osteoporos Sarcopenia 5:69–77
Rizzoli R (2022) Dairy products and bone health. Aging Clin Exp Res 34:9–24
Cummings SR, Nevitt MC (1989) A hypothesis: the causes of hip fractures. J Gerontol 44:M107-111
Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, Lamb SE (2012) Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2012(9):CD007146. https://doi.org/10.1002/14651858.CD007146.pub3
Bhasin S, Gill TM, Reuben DB et al (2020) A randomized trial of a multifactorial strategy to prevent serious fall injuries. N Engl J Med 383:129–140
Lamb SE, Bruce J, Hossain A et al (2020) Screening and intervention to prevent falls and fractures in older people. N Engl J Med 383:1848–1859
Guirguis-Blake JM, Michael YL, Perdue LA, Coppola EL, Beil TL (2018) Interventions to prevent falls in older adults: updated evidence report and systematic review for the US preventive services task force. JAMA 319:1705–1716
Crandall CJ, Newberry SJ, Diamant A, Lim YW, Gellad WF, Booth MJ, Motala A, Shekelle PG (2014) Comparative effectiveness of pharmacologic treatments to prevent fractures: an updated systematic review. Ann Intern Med 161:711–723
Black DM, Cummings SR, Karpf DB et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group Lancet 348:1535–1541
McClung MR, Geusens P, Miller PD et al (2001) Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 344:333–340
Black DM, Delmas PD, Eastell R et al (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Kendler DL, Marin F, Zerbini CAF et al (2018) Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 391:230–240
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A (2017) Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 377:1417–1427
McClung MR (2021) Role of bone-forming agents in the management of osteoporosis. Aging Clin Exp Res 33:775–791
Cosman F, Nieves JW, Dempster DW (2017) Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res 32:198–202
Kanis JA, Cooper C, Rizzoli R et al (2017) Identification and management of patients at increased risk of osteoporotic fracture: outcomes of an ESCEO expert consensus meeting. Osteoporos Int 28:2023–2034
Kanis JA, Harvey NC, McCloskey E et al (2020) Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int 31:1–12
Kanis JA, Dawson A, Oden A, Johnell O, de Laet C, Jonsson B (2001) Cost-effectiveness of preventing hip fracture in the general female population. Osteoporos Int 12:356–361
Rossouw JE, Anderson GL, Prentice RL et al (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333
Anderson GL, Limacher M, Assaf AR et al (2004) Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 291:1701–1712
Cauley JA, Robbins J, Chen Z et al (2003) Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA 290:1729–1738
McCloskey EV, Beneton M, Charlesworth D et al (2007) Clodronate reduces the incidence of fractures in community-dwelling elderly women unselected for osteoporosis: results of a double-blind, placebo-controlled randomized study. J Bone Miner Res 22:135–141
McCloskey EV, Johansson H, Oden A, Vasireddy S, Kayan K, Pande K, Jalava T, Kanis JA (2009) Ten-year fracture probability identifies women who will benefit from clodronate therapy–additional results from a double-blind, placebo-controlled randomised study. Osteoporos Int 20:811–817
McCloskey E (2016) A BMD threshold for treatment efficacy in osteoporosis? A need to consider the whole evidence base. Osteoporos Int 27:417–419
National Institute for Clinical Excellence NICE (2005) Technology appraisal guidance 87. Bisphosphonates (alendronate, etidronate, risedronate), selective oestrogen receptor modulators (raloxifene) and parathyroid hormone (teriparatide) for the secondary prevention of osteoporotic fragility fractures in postmenopausal women. National Institute for Clinical Excellence, London
NICE (2008) TA161 (revised) Raloxifene and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women. National Institute for Health and Clinical Excellence. https://www.nice.org.uk/guidance/ta161. Accessed 14 August 2021
NICE (2008) TA160. Raloxifene for the primary prevention of osteoporotic fragility fractures in postmenopausal women. https://www.nice.org.uk/guidance/ta160. Accessed 19 May 2022
NICE (2008) TA161 Alendronate, etidronate, risedronate, raloxifene, strontium ranelate and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women. https://www.nice.org.uk/guidance/ta161. Accessed 14 August 2021
NICE (2010) Denosumab for the prevention of osteoporotic fractures in postmenopausal women. https://www.nice.org.uk/guidance/ta204. Accessed 19 May 2022
National Institute for Health and Care Excellence (2017) Bisphosphonates for treating osteoporosis. https://www.nice.org.uk/guidance/ta464/resources/bisphosphonates-for-treating-osteoporosis-pdf-82604905556677. Updated: Jul 8, 2019. Accessed 18 Aug 2021
British Orthopaedic Association (2007) The care of patients with fragility fracture. Available at: http://www.boaacuk/Publications/Documents/The%20Care%20of%20Patients%20with%20Fragility%20Fracturepdf. Accessed 18 Aug 2021
NICE (2011) Hip fracture: management. Clinical guideline CG124. Published: 22 June 2011 (Updated May 2017) www.nice.org.uk/guidance/cg124. Accessed 18 Aug 2021
Neuburger J, Currie C, Wakeman R, Tsang C, Plant F, De Stavola B, Cromwell DA, van der Meulen J (2015) The impact of a national clinician-led audit initiative on care and mortality after hip fracture in England: an external evaluation using time trends in non-audit data. Med Care 53:686–691
Chesser TJS, Inman D, Johansen A et al (2020) Hip fracture systems-European experience. OTA Int 3:e050
Marsh D, Akesson K, Beaton DE et al (2011) Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int 22:2051–2065
Akesson K, Marsh D, Mitchell PJ, McLellan AR, Stenmark J, Pierroz DD, Kyer C, Cooper C, Group IOFFW (2013) Capture the fracture: a best practice framework and global campaign to break the fragility fracture cycle. Osteoporos Int 24:2135–2152
Majumdar SR, McAlister FA, Johnson JA et al (2018) Comparing strategies targeting osteoporosis to prevent fractures after an upper extremity fracture (C-STOP Trial): a randomized controlled trial. J Bone Miner Res 33:2114–2121
Majumdar SR, Johnson JA, McAlister FA, Bellerose D, Russell AS, Hanley DA, Morrish DW, Maksymowych WP, Rowe BH (2008) Multifaceted intervention to improve diagnosis and treatment of osteoporosis in patients with recent wrist fracture: a randomized controlled trial. CMAJ 178:569–575
Majumdar SR, Rowe BH, Folk D et al (2004) A controlled trial to increase detection and treatment of osteoporosis in older patients with a wrist fracture. Ann Intern Med 141:366–373
Javaid MK (2021) Efficacy and efficiency of fracture liaison services to reduce the risk of recurrent osteoporotic fractures. Aging Clin Exp Res 33:2061–2067
Axelsson KF, Johansson H, Lundh D, Moller M, Lorentzon M (2020) Association between recurrent fracture risk and implementation of fracture liaison services in four swedish hospitals: a cohort study. J Bone Miner Res 35:1216–1223
McCloskey E, Rathi J, Heijmans S et al (2021) The osteoporosis treatment gap in patients at risk of fracture in European primary care: a multi-country cross-sectional observational study. Osteoporos Int 32:251–259
Leroy S, Saunders-Hastings P, Eusebi P, Taieb V, Abrahamsen B, McCloskey EV, Fujiwara S, Libanati C, Moayyeri A (2022) Treatment gap among patients with primary osteoporosis: a systematic literature review and meta-analysis. Osteoporosis International 32 (Suppl 1) (https://doi.org/10.1007/s00198-021-06125-9):S189
Bussell ME (2021) Improving bone health: addressing the burden through an integrated approach. Aging Clin Exp Res 33:2777–2786
Rubin KH, Rothmann MJ, Holmberg T et al (2018) Effectiveness of a two-step population-based osteoporosis screening program using FRAX: the randomized Risk-stratified Osteoporosis Strategy Evaluation (ROSE) study. Osteoporos Int 29:567–578
Merlijn T, Swart KM, van Schoor NM et al (2019) The effect of a screening and treatment program for the prevention of fractures in older women: a randomized pragmatic trial. J Bone Miner Res 34:1993–2000
Rubin KH, Holmberg T, Rothmann MJ et al (2015) The risk-stratified osteoporosis strategy evaluation study (ROSE): a randomized prospective population-based study. Design and baseline characteristics. Calcif Tissue Int 96:167–179
Merlijn T, Swart KMA, Netelenbos JC, Elders PJM (2020) Reply to “Screening for high fracture risk.” Osteoporos Int 31:1183–1184
Merlijn T, Swart KMA, van der Horst HE, Netelenbos JC, Elders PJM (2020) Fracture prevention by screening for high fracture risk: a systematic review and meta-analysis. Osteoporos Int 31:251–257
McCloskey EV, Lenaghan E, Clarke S, et al. (2016) Screening based on FRAX fracture risk assessment reduces the incidence of hip fractures in older community-dwelling women – results from the SCOOP study in the UK Journal of Bone and Mineral Research 31(S1):S40. https://www.asbmronlinelibrarywileycom/doi/epdf/101002/jbmr3107. Accessed 18 Aug 2021
McCloskey EV, Harvey NC, Johansson H, Lorentzon M, Vandenput L, Liu E, Kanis JA (2021) Global impact of COVID-19 on non-communicable disease management: descriptive analysis of access to FRAX fracture risk online tool for prevention of osteoporotic fractures. Osteoporos Int 32:39–46
Njeh CF, Fuerst T, Hans D, Blake GM, Genant HK (1999) Radiation exposure in bone mineral density assessment. Appl Radiat Isot 50:215–236
Torgerson DJ, Thomas RE, Campbell MK, Reid DM (1997) Randomized trial of osteoporosis screening. Use of hormone replacement therapy and quality-of-life results. Arch Intern Med 157:2121–2125
Reventlow SD, Hvas L, Malterud K (2006) Making the invisible body visible. Bone scans, osteoporosis and women’s bodily experiences. Soc Sci Med 62:2720–2731
Richardson JC, Hassell AB, Hay EM, Thomas E (2002) “I’d rather go and know”: women’s understanding and experience of DEXA scanning for osteoporosis. Health Expect 5:114–126
Black DM, Geiger EJ, Eastell R, Vittinghoff E, Li BH, Ryan DS, Dell RM, Adams AL (2020) Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N Engl J Med 383:743–753
Shane E, Burr D, Abrahamsen B et al (2014) Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 29:1–23
Khosla S, Burr D, Cauley J et al (2007) Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 22:1479–1491
van de Laarschot DM, McKenna MJ, Abrahamsen B, Langdahl B, Cohen-Solal M, Guanabens N, Eastell R, Ralston SH, Zillikens MC (2020) Medical management of patients after atypical femur fractures: a systematic review and recommendations from the European Calcified Tissue Society. J Clin Endocrinol Metab 105:682–1689
Abrahamsen B, Eiken P, Prieto-Alhambra D, Eastell R (2016) Risk of hip, subtrochanteric, and femoral shaft fractures among mid and long term users of alendronate: nationwide cohort and nested case-control study. BMJ 353:i3365
Barr RJ, Stewart A, Torgerson DJ, Reid DM (2010) Population screening for osteoporosis risk: a randomised control trial of medication use and fracture risk. Osteoporos Int 21:561–568
Soreskog E, Borgstrom F, Shepstone L et al (2020) Long-term cost-effectiveness of screening for fracture risk in a UK primary care setting: the SCOOP study. Osteoporos Int 31:1499–1506
Owen L, Pennington B, Fischer A, Jeong K (2017) The cost-effectiveness of public health interventions examined by NICE from 2011 to 2016. Journal of Public Health | Vol 40, No 3, pp 557–566 | 101093/pubmed/fdx119 40:557–566 (510.1093/pubmed/fdx1119)
Pharoah PD, Sewell B, Fitzsimmons D, Bennett HS, Pashayan N (2013) Cost effectiveness of the NHS breast screening programme: life table model. BMJ 346:f2618
Lee D, Muston D, Sweet A, Cunningham C, Slater A, Lock K (2010) Cost effectiveness of CT colonography for UK NHS colorectal cancer screening of asymptomatic adults aged 60–69 years. Appl Health Econ Health Policy 8(13):141–154
Department of Health (2009) Falls and fractures: effective interventions in health and social care. https://www.laterlifetraining.co.uk/wp-content/uploads/2011/12/FF_Effective-Interventions-in-health-and-social-care.pdf. Accessed 19 May 2022
Condurache CI, Chiu S, Chotiyarnwong P et al (2020) Screening for high hip fracture risk does not impact on falls risk: a post hoc analysis from the SCOOP study. Osteoporos Int 31:457–464
Solutions for Public Health (2019) Screening for osteoporosis in postmenopausal women. External review against programme appraisal criteria for the UK National Screening Committee. https://view-health-screening-recommendations.service.gov.uk/osteoporosis/. Accessed 18 Aug 2021
UK National Screening Committee (2019) Screening for Osteoporosis in Postmenopausal Women 08 November 2019. https://view-health-screening-recommendations.service.gov.uk/osteoporosis/. Accessed 18 Aug 2021
EUnetHTA OTCA19 Assessment Team. (2019) Screening for osteoporosis in the general population. Collaborative Assessment. Diemen (The Netherlands): EUnetHTA; 2019. Report No.: OTCA19. Available from https://www.eunethta.eu. Accessed 18 Aug 2021
Gail MH, Pfeiffer RM (2018) Breast cancer risk model requirements for counseling, prevention, and screening. J Natl Cancer Inst 110:994–1002
Clark EM, Gould V, Morrison L, Ades AE, Dieppe P, Tobias JH (2012) Randomized controlled trial of a primary care-based screening program to identify older women with prevalent osteoporotic vertebral fractures: Cohort for Skeletal Health in Bristol and Avon (COSHIBA). J Bone Miner Res 27:664–671
Acknowledgements
This position paper was prepared by the International Osteoporosis Foundation Epidemiology/Quality of Life Working Group and has been endorsed by the International Osteoporosis Foundation. We are grateful for administrative support from Dr Dominique Pierroz.
Funding
Work informing this project was supported by the UK Medical Research Foundation (MRF-145–0011-DG-HARV-C0913). N.C.H., E.M.C., E.M.D. and C.C. are supported by the UK Medical Research Council (MC_PC_21003; MC_PC_21001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This narrative article contains no original data and thus issues of ethics, informed consent and patient confidentiality do not apply.
Conflict of interest
E.V.M reports consultant/advisor fees, speaker honoraria and or, research funding from AgNovos, Amgen, Consilient Healthcare, Fresenius Kabi, Gedeon Richter, Internis, Lilly, Novartis, ObsEva, Synexus and UCB, all outside the submitted work. E.M.C. reports lecture fees and travel support from Eli Lilly, Pfizer and UCB, outside the submitted work. N.C.H. reports personal fees, consultancy, lecture fees and honoraria from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, UCB, Kyowa Kirin, Servier, Shire, Consilient Healthcare and Internis Pharma, outside the submitted work. J.A.K. reports a grant from UCB, outside the submitted work. He is the architect of FRAX® but has no financial interest. C.C. reports personal fees from ABBH, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier and Takeda, outside the submitted work. B.A. reports grants and personal fees from UCB, personal fees from Amgen, grants from Novartis, grants and personal fees from Pharma-cosmos, grants and personal fees from Kyowa Kirin, personal fees for Gedeon Richter outside the submitted work. BA also serves on the NovoNordisk Foundation Grants Committee on Endocrinology and Metabolism. M.L. reports lecture fees from Amgen, Astellas, Lilly, Meda, Renapharma and UCB Pharma and consulting fees from Amgen, Radius Health, UCB Pharma, Renapharma and Consilient Health, outside the submitted work. D.P-A. reports institutional receipt of speaker fees from AMGEN and UCB Biopharma; consultancy fees from UCB Biopharma; research grants from UCB, Amgen, and Les Laboratoires Servier, all outside of the submitted work. J. A. reports grants and personal fees from Amgen outside the submitted work, grants from Radius and has been an advisor to Gilead and is part of their speaker’s bureau, outside the submitted work. F.B. is employed and is a shareholder in Quantify Research, a health economic research consultancy, outside of the submitted work. O.B. reports grant support from IBSA, MSD, Nutraveris, Novartis, Pfizer, Rottapharm, Servier and Theramex; consulting or lecture fees from Bayer, Genevrier, IBSA, Rottapharm, Servier, SMB and TRB Chemedica; all outside the submitted work. All other authors have no relevant conflicts of interest in relation to the submitted work.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
P. Chotiyarnwong and E. V. McCloskey are joint first authors
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chotiyarnwong, P., McCloskey, E.V., Harvey, N.C. et al. Is it time to consider population screening for fracture risk in postmenopausal women? A position paper from the International Osteoporosis Foundation Epidemiology/Quality of Life Working Group. Arch Osteoporos 17, 87 (2022). https://doi.org/10.1007/s11657-022-01117-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-022-01117-6