Abstract
Summary
The coordination of Fracture Liaison Services (FLS) with Primary Care (PC) is necessary for the continuity of care of patients with fragility fractures. This study proposes a Best Practice Framework (BPF) and performance indicators for the implementation and follow-up of FLS-PC coordination in clinical practice in Spain.
Purpose
To develop a BPF for the coordination of FLS with PC in Spain and to improve the continuity of care for patients with fragility fractures.
Methods
A Steering Committee selected experts from seven Spanish FLS and related PC doctors and nurses to participate in a best practice workshop. Selection criteria were an active FLS with an identified champion and prior contact with PC centres linked to the hospital. The main aim of the workshop was to review current FLS practices in Spain and their integration with PC. A BPF document with processes, tools, roles, and metrics was then generated.
Results
Spanish FLS consists of a multidisciplinary team of physicians/nurses but with low participation of other professionals and PC staff. Evaluation and treatment strategies are widely variable. Four desired standards were agreed upon: (1) Effective channels for FLS-PC communication; (2) minimum contents of an FLS clinical report and its delivery to PC; (3) adherence monitoring 3 months after FLS baseline visit; and (4) follow-up by PC. Proposed key performance indicators are (a) number of FLS-PC communications, including consensus protocols; (b) confirmation FLS report received by PC; (c) medical/nursing PC appointment after FLS report received; and (d) number of training sessions in PC.
Conclusions
The BPF provides a comprehensive approach for FLS-PC coordination in Spain, to promote the continuity of care in patients with fragility fractures and improve secondary prevention. The implementation of BPF recommendations and performance indicator tracking will benchmark best FLS practices in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis and its associated fragility fractures are globally common conditions contributing significantly to morbidity, mortality, and healthcare spending, and thus constitute a major public health problem [1].
According to recent statistics from the International Osteoporosis Foundation, worldwide, 1 in 3 women and 1 in 5 men over the age of 50 will experience fragility fractures in their lifetime [2]. In Spain, it was estimated in 2013 that a total of 552,879 women and 161,922 men would suffer fragility fractures in the next 10 years [3].
Patients experiencing the first fragility fracture are at a significantly higher risk of subsequent fractures [4]. Peri- and postmenopausal women with low trauma fracture have approximately twice the risk of subsequent fractures compared with similar patients without fracture [5]. Moreover, osteoporosis treatments are more cost-effective when prescribed to older adults with prior fracture [6]. Thus, clinicians caring for fracture patients must consider options for secondary fracture prevention. In patients at moderate fracture risk, bone mineral density can help guide therapeutic decisions. In interventional studies aimed at improving osteoporosis management after a fracture, bone densitometry was used in a median of 43% of patients [7]. However, in Spain, the use of bone densitometry (Dual energy X-ray absorciometry) for fracture risk assessment is limited [8]. Moreover, despite the availability of medications proven to reduce the risk of further fractures [9], fewer than 20% of individuals who sustain a fragility fracture receive such therapies within the first year following the fracture [10, 11]. This results in a pervasive worldwide treatment and strategy gap for secondary fracture prevention [11, 12]. There are multiple contributors to this large gap, such as clinicians failing to adhere to treatment guidelines [11], the low priority assigned to secondary fracture prevention by primary care (PC) and hospital physicians [13], and poor patient adherence to treatment [11].
Faced with this situation, in 2011, the Fracture Working Group of the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF) published a position paper on coordinator-based systems for secondary prevention in fragility fracture patients. The paper consolidated knowledge on the development, effectiveness, and common factors that underpin successful clinical systems designed to close the secondary fracture prevention care gap [14]. Fracture Liaison Services (FLSs) are care coordinator-based secondary fracture prevention programmes that systematically identify fragility fracture patients, then assess, investigate, and treat them for underlying osteoporosis as appropriate [15]. They are a cost-effective strategy for reducing the osteoporosis care gap, refracture rate, and mortality [16, 17]. A nurse coordinator or other professional acts as liaison between the patient, hospital team, and PC physician, to ensure continuity of care [13]. If effective communication between the FLS and PC is established, PC physicians are well placed and willing to manage osteoporosis care in the longer term [12].
However, in the current scenario in Spain, once the fracture has healed, there is no clear reference as to who should undertake the patient’s subsequent follow-up and care [18]. Existing models for secondary fracture prevention mostly come from Anglo-Saxon countries [14, 19,20,21] and must be adapted to the specific local healthcare environment [18].
The aim of this project was to develop a Best Practice Framework (BPF) for FLS-PC coordination in Spain, to guarantee the continuity of care for patients with fragility fractures.
Methods
The Spanish Society for Research on Bone and Mineral Metabolism (SEIOMM) supported a workshop to define and standardise processes, tools, roles, and metrics for FLS-PC coordination.
A literature review regarding current FLS practices in Spain was performed to inform the workshop. Searches were made in international (PubMed) and national databases (MEDES, IBECS), as well as grey literature (research published in non-commercial form, e.g. reports, conference proceedings, or doctoral theses/dissertations), up until December 2017. The search strategy focused on the pathology of interest (“osteoporosis”), fracture as the main complication, and treatment management in FLS (Supplementary Table 1).
A Steering Committee was created with champions from three FLS centres in Spain and the UK (Hospital Dr. Negrín, n = 2; NDORMS, n = 1). Champions (n = 9) and case managers (n = 1) from seven consolidated Spanish FLS were invited to participate in a workshop, together with their related PC doctors (n = 11) and nurses (n = 8) (Supplementary Table 2). Selection criteria for FLS participation were to have an active FLS, with a well-identified champion, and to have prior contact with the PC centres linked to the hospital.
During the workshop, each champion presented current practices and connection model with PC in their healthcare area. The FLS best practices followed by the Steering Committee, and information derived from the literature review, were presented as key indicators of the performance and coordination of the FLS with PC.
Two discussion groups were requested to reach a consensus on the relationship between FLS and PC. One group focused on the communication between the FLS and PC, to ensure the clinical report is complete and reaches the PC centre. The second group focused on the coordination of PC doctors and nurses when they receive the clinical report, and coordination with the hospital for patient follow-up. During the first round of discussions, the Steering Committee posed questions to define best practice standards and how they should be measured. During the second round, conclusions from each discussion group were debated. The experts then generated the first draft of the BPF, reviewed the draft, made suggestions, and approved the final version of the BPF for FLS-PC coordination in Spain. The BPF, which includes the recommendations proposed and performance indicators, is presented below.
Results
Current practices in Spanish FLS
Current practices in Spanish FLS and their coordination with PC are presented (Table 1), considering a Spanish excellence FLS (FLS of the Hospital Dr. Negrín, a national reference with vast expertise) and the other seven FLS participating in the study (Supplementary Table 2).
FLS composition and patient identification
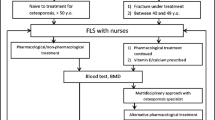
FLS consist of a multidisciplinary team, mainly comprised by physicians from different specialties and nurses (Fig. 1). Each FLS unit identifies patients with fragility fracture through several pathways. The main one is the emergency list, while other less common modes include patient lists from rheumatology services, orthogeriatrics, or PC.
Type of fractures included
All FLS include hip fracture, and most of them include vertebral fracture, and fracture of the radius and proximal humerus.
Evaluation
All FLS request a blood test to evaluate the patient’s condition. Most of them also collect densitometry and an X-ray of the spine. Data collected to a lesser extent are Fracture Risk Assessment Tool (FRAX), nutritional assessment, fall risk scale, trabecular bone score, or assessment of functional capacity.
Intervention
The FLS follow different scientific criteria to determine the intervention and prescription recommendations for secondary fracture prevention, including the NOF [22] and SEIOMM [23] criteria. All FLS recommend pharmacological treatment when appropriate; and most of them give nutritional advice; make recommendations about lifestyle and calcium and vitamin D supplements; or include a fall prevention service. Recommendations on gait rehabilitation, physical exercise, or occupational therapy are less common.
Follow-up
In most FLS (75.0%), patient follow-up is undertaken at hospital visits face-to-face with the specialist (orthopaedics, geriatricians, rheumatologists, and internal medicine physicians). Time to the first follow-up visit varies among centres, ranging from 1 month to 2 years, as well as the number of visits carried out, from none to three in a year. In 37.5% of FLS, general follow-up is also performed by the PC physician, while in 25.0% of FLS, follow-up is exclusively performed in PC.
In some FLS, the patient is also monitored by telephone to assess treatment adherence, and only the Spanish excellence FLS also monitors treatment adherence through an electronic prescription platform.
Coordination with PC
Three-quarters of FLS have an established pathway for continuous coordination with PC, whether through e-mail/telephone/fax or virtual consultations. However, a designated individual to manage this coordination is available only in 25.0% of FLS, being a support technician or a case manager nurse.
Another means of communication with PC is the clinical report sent to the PC physician. In 83.3% of the cases, the report is sent directly to the PC physician or delivered via the patient, and in 33.3% of cases, the report is shared by common software used by the hospital and PC.
Only 25.0% of FLS deliver training sessions at PC centres to emphasise the importance of secondary prevention, treatment adherence, and understanding the report issued by the FLS.
BPF standards for the coordination of FLS with PC in Spain
Four standards were agreed upon: (1) Effective communication channels between the FLS and PC; (2) minimum contents of the FLS clinical report and its delivery to PC; (3) treatment adherence 3 months after the visit to FLS; and (4) follow-up by PC doctor and nurse. Performance indicators proposed were (a) number of FLS-PC communications, including consensus protocols; (b) confirmation of the FLS clinical report reception by PC; (c) medical and nursing appointments in PC after the FLS report; and (d) training sessions received in PC (Table 2).
Discussion
The osteoporosis care gap after fragility fractures is growing substantially. The reason this care gap exists, and persists, is multifactorial in nature [15]. Lack of clarity regarding where clinical responsibility lies has been identified as a major contributing factor [24]. Although initiating treatment in the orthopaedic department and then delegating care to PC physicians has been recommended [25], simply delegating care to PC does not positively influence provision of appropriate preventative measures nor does it improve subsequent fracture prevention. This is because neither orthopaedic surgeons who treat acute fractures nor PC physicians who provide long-term healthcare appear to be engaged in secondary prevention [26, 27]. It is therefore important to motivate PC physicians to become more involved in the management of low-impact fractures [26]. International recommendations for secondary fracture prevention advocate that processes should be in place to ensure reliable provision of long-term management of fracture risk. In healthcare systems with established PC infrastructure, local PC must be involved in developing the processes that they will implement for post-fracture care [15].
It is encouraging that Spain is actively creating FLS for secondary fracture prevention [28]. Spanish FLS participating in this study share common characteristics and patterns, such as their multidisciplinary composition, type of fractures treated, and main pathways for fracture-patient identification. However, they differ significantly in the extent of evaluation, outreach of intervention strategies, and the frequency and routes of patient follow-up. Moreover, several improvement opportunities exist in the coordination with PC.
As previously described, the implementation of FLS may be limited by a lack of PC participation [26]. In this context, standardised practices are needed to achieve effective coordination between newly created FLS and PC, and thus improve the continuity of care of patients with fragility fractures.
In general, there is limited information regarding how the coordination between FLS and PC is performed and little data about the performance of this communication. Chang et al. [29] identified several treatment gaps in current FLS and provided recommendations for best practice establishment of future FLS across the Asia-Pacific region. Their findings emphasise the importance of PC physicians continuing to prescribe treatment and ensure service remains convenient. In current practice worldwide, specialists rely on PC to manage osteoporosis. However, PC doctors routinely do so only if advised by specialists, and osteoporosis experts—usually endocrinologists or rheumatologists—have no reason to interact with the patient post-fracture [28]. The involvement of other specialists, such as geriatricians, in the acute care of elderly patients with fractures (mainly hip fractures) in “Orthogeriatric Units”, has shown to improve secondary fracture prevention [30]. Another proven solution to close the secondary fracture prevention care gap is to establish an FLS [15], necessarily coordinated with PC, highlighting the need for the development of a BPF for this coordination.
In the present study, four BPF standards were proposed to address the main needs for FLS-PC coordination in Spain: (1) Promotion of FLS-AP communication, (2) unification of FLS clinical report metrics, (3) systematic control of the adherence to treatment by the FLS, and (4) improvement of patient follow-up by PC.
The four standards we propose have previously been reported as relevant issues in osteoporosis management. The FLS coordinator, who takes care of all aspects of the process (patient identification, investigation, and therapeutic intervention), is responsible for providing adequate medical information to PC physicians. FLS experts proposed seven possible general communication channels, ranging from face-to-face meetings to telecommunication channels, such as the telephone or e-mail, in line with general literature regarding communication systems in healthcare [31]. An important secondary fracture prevention strategy relies on the FLS report to PC with treatment recommendations [7]. Our BPF recommends which items to include in this report and possible ways to deliver it to PC. Medication adherence remains a particular challenge in osteoporosis [32], with little consensus on how to identify non-adherent patients [33,34,35]. Our BPF recommends checking patient adherence to treatment in the first 3–4 months after the indication of treatment, by both telephone call and electronic receipt.
For BPF implementation in the seven FLS and their PC centres participating in the project, a training plan for PC will be designed. It will encompass basic information (osteoporosis, secondary prevention) and the BPF standards. Training sessions will be delivered by the champion of the FLS and a defined case manager of the PC centre. In this regard, providing PC physicians with information on fracture risk, osteoporosis, and appropriate preventative measures in discharge reports has proven to be an important part of secondary fracture prevention [17]. Afterwards, a case report form will be designed according to the metrics established in the workshop to monitor the implementation and performance of the BPF standards. The performance indicators will be recorded quarterly by the FLS champion and nurse/case manager of each FLS and PC centre.
Our proposed BPF will serve as a model for the creation of new FLS and as a guide for improving existing ones. Adherence to the recommendations in our BPF may improve the management and follow-up of patients with fragility fracture. Overall, the percentage of patients suffering from a new fracture could be reduced and, therefore, the direct and indirect costs associated with the fragility fracture lowered.
Limitations of the study
The study was conducted from the perspective of Spanish healthcare professionals and the results might not be extrapolated to other countries. However, the development of standards for the coordination FLS-PC in Spain is crucial, given the increasing number of FLS created, and the results of the study provide a guide for the optimal long-term management of patients with fragility fractures. Further follow-up must be undertaken to demonstrate the efficiency of the BPF in increasing the compliance and long-term rate of therapy utilisation.
Conclusions
Local and national prevention strategies must be put in place rapidly to reverse the increasing number of fragility fractures occurring in Spain. The BPF provides a comprehensive approach for the coordination between FLS and PC in Spain, to promote the continuity of care in patients with fragility fractures and improve secondary prevention. The implementation of the BPF in clinical practice will provide feedback for ongoing improvement of BPF standards and to benchmark the best FLS in the future.
References
Curtis EM, Moon RJ, Harvey NC, Cooper C (2017) The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone 104:29–38. https://doi.org/10.1016/j.bone.2017.01.024
International Osteoporosis Foundation. Osteoporosis facts and statistics. Available from: https://www.iofbonehealth.org/facts-and-statistics/. Accessed July 2019
González López-Valcárcel B, Sosa Henríquez M (2013) Estimate of the 10-year risk of osteoporotic fractures in the Spanish population. Med Clin (Barc) 140:104–109
Sözen T, Özışık L, Başaran NÇ (2017) An overview and management of osteoporosis. Eur J Rheumatol 4:46–56
Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA 3rd, Berger M (2000) Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical analysis. J Bone Miner Res 15:721–739
Sáez-López P, Etxebarria-Foronda I, Mesa Lampre M, Alonso García N, Sánchez HN (2019) Efficacy, cost, and aspects to take into account in the treatment of osteoporosis in the elderly. Rev Esp Geriatr Gerontol 54:156–167
Sale JEM, Beaton D, Posen J, Elliot-Gibson V, Bogoch E (2011) Systematic review on interventions to improve osteoporosis investigation and treatment in fragility fracture patients. Osteoporos Int 22:2067–2082
Gómez-Vaquero C, Bianchi M, Santo P, Roig-Vilaseca D, Narváez J, Nolla JM (2012) The activity of a Spanish bone densitometry unit revisited under the point of view of FRAX. Reumatol Clin 8:179–183
Chan D, Chang L, Akesson K, Mitchell P, Chen C, Lewiecki E et al (2018) Consensus on best practice standards for Fracture Liaison Service in the Asia-Pacific region. Arch Osteoporos 13:59
Kanis J, Svedbom A, Harvey N, McCloskey E (2014) The osteoporosis treatment gap. J Bone Miner Res 29:1926–1928
Kanis JA, Cooper C, Rizzoli R, Abrahamsen B, Al-Daghri NM, Brandi ML et al (2017) Identification and management of patients at increased risk of osteoporotic fracture: outcomes of an ESCEO expert consensus meeting. Osteoporos Int 28:2023–2034
Harvey N, McCloskey E, Mitchell P, Dawson-Hughes B, Pierroz D, Reginster J, Rizzoli R, Cooper C, Kanis JA (2017) Mind the (treatment) gap: a global perspective on current and future strategies for prevention of fragility fractures. Osteoporos Int 28:1507–1529
Eisman J, Bogoch E, Dell R, Harrington J, McKinney RJ, Mclellan A et al (2012) Making the first fracture the last fracture : ASBMR task force report on secondary. JBMR. 27:1–8
Marsh D, Akesson K, Beaton D, Bogoch E, Boonen S, Brandi M, McLellan A, Mitchell PJ, Sale JE, Wahl DA, IOF CSA Fracture Working Group (2011) Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int 22:2051–2065
Åkesson K, Marsh D, Mitchell PJ, Mclellan AR, Stenmark J, Pierroz D et al (2013) Capture the fracture: a best practice framework and global campaign to break the fragility fracture cycle. Osteoporos Int 24:2135–2152
Briot K (2017) Fracture liaison services. Curr Opin Rheumatol 29:416–421
Wu CH, Tu ST, Chang YF, Chan DC, Chien JT, Lin CH, Singh S, Dasari M, Chen JFTK (2017) Fracture liaison services improve outcomes of patients with osteoporosis-related fractures: a systematic literature review and meta-analysis. Osteoporos Sarcopen 3:S51–S52
Naranjo A, Ojeda-Bruno S, Bilbao Cantarero A, Quevedo Abeledo JC, Henríquez-Hernández LA, Rodríguez-Lozano C (2014) Results of a model of secondary prevention for osteoporotic fracture coordinated by rheumatology and focused on the nurse and primary care physicians. Reumatol Clin 10:299–303
Ganda K, Puech M, Chen JS, Speerin R, Bleasel J, Center JR (2013) Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int 24(2):393–406
McLellan A, Gallacher S, Fraser M, McQuillian C (2003) The fracture liaison service: success of a program for the evaluation and management of patients with osteoporotic fracture. Osteoporos Int 14:1028–1034
Harrington JT, Barash HL, Day S, Lease J (2004) Redesigning the care of fragility fracture patients to improve osteoporosis management: a health care improvement project. 53:198–204
National Osteoporosis Foundation (2008) Clinician’s guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation, Washington DC
Working Group of the Clinical Practice Guide on Osteoporosis and Fragility Fracture Prevention. Clinical practice guide on osteoporosis and prevention of fragility fractures. Quality Plan for the National Health System of the Ministry of Health and Social [Internet]. Available from: https://portal.guiasalud.es/wpcontent/uploads/2018/12/GPC_476_Osteoporosis_AIAQS_compl_ant.pdf. Accessed June 2019
Elliot-Gibson V, Bogoch ER, Jamal SA, Beaton DE (2004) Practice patterns in the diagnosis and treatment of osteoporosis after a fragility fracture: a systematic review. Osteoporos Int 15:767–778
Hoang-Kim A, Schemitsch E, Sale JE, Beaton D, Warmington K, Kulkarni AV, Reeves S (2014) Understanding osteoporosis and fractures: an introduction to the use of qualitative research. Arch Orthop Trauma Surg 134(2):207–217
Vaculik J, Stepan JJ, Dungl P, Majerní M, Alexander Č (2017) Secondary fracture prevention in hip fracture patients requires cooperation from general practitioners. Arch Osteoporos 12(1):49
Harrington JT, Broy SB, Derosa AM, Licata AA, Shewmon DA (2002) Hip fracture patients are not treated for osteoporosis: a call to action. Arthritis Rheum 47:651–654
International Osteoporosis Foundation. Capture the fracture. [Internet]. Available from: http://www.capturethefracture.org. Accessed June 2019
Chang Y, Huang C, Hwang J, Kuo J, Lin K, Huang H et al (2018) Fracture liaison services for osteoporosis in the Asia-Pacific region: current unmet needs and systematic literature review. Osteoporos Int 29:779–792
Sabharwal S, Wilson H (2015) Orthogeriatrics in the management of frail older patients with a fragility fracture. Osteoporos Int 26:2387–2399
Coiera E (2006) Communication systems in healthcare. Clin Biochem Rev 27:89–98
Javaid MK, Kyer C, Mitchell PJ, Chana J, Moss C, Edwards MH (2015) Effective secondary fracture prevention: implementation of a global benchmarking of clinical quality using the IOF Capture the Fracture® Best Practice Framework tool. Osteoporos Int 26:2573–2578
Burch J, Rice S, Yang H, Neilson A, Stirk L, Francis R et al (2014) Systematic review of the use of bone turnover markers for monitoring the response to osteoporosis treatment: the secondary prevention of fractures, and primary prevention of fractures in high-risk groups. Health Technol Assess 18:1–180
Vasikaran S, Cooper C, Griesmacher A, Morris HA, Trenti T, Kanis JA et al (2011) International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine Position on bone marker standards in osteoporosis. Clin Chem Lab Med 49:1271–1274
Bell KJL, Hayen A, Macaskill P, Irwig L, Craig JC, Ensrud K et al (2009) Value of routine monitoring of bone mineral density after starting bisphosphonate treatment : secondary analysis of trial data. BMJ. 338:b2266
Acknowledgments
This study was designed in collaboration with Neus Vidal and Clara Gabás, from Outcomes′10. Writing assistance was supported by Amgen S.A. and provided by Clara Gabás, PhD, from Outcomes′10.
Funding
The study was sponsored by Amgen S.A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
Naranjo A has received research grants from Amgen, consulting fees from UCB and has participated in speakers’ bureaus for Amgen and Lilly. Ojeda S has received research grants from Amgen. Canals L and Balcells-Oliver M work at Amgen and hold stock in Amgen. Mora-Fernández J has received research grants from Amgen and consulting fees from Amgen, Lilly, and UCB-Pharma. Lladó B has received fees as a speaker at medical sessions and meetings for Amgen and UCB, and as a speaker at medical sessions for Lilly. Olmo FJ has received consulting fees from Amgen, UCB and Abbott. Prieto-Alhambra D has received research Grants from Amgen, UCB Biopharma and Les Laboratoires Servier, and non-remunerative positions; the department has received fees for consultancy services from UCB Biopharma and for speaker and advisory board membership services from Amgen. Giner M, Cancio JM, Duaso E, Montoya MJ, Pablos C, González A, and Menéndez A declare no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naranjo, A., Ojeda, S., Giner, M. et al. Best Practice Framework of Fracture Liaison Services in Spain and their coordination with Primary Care. Arch Osteoporos 15, 63 (2020). https://doi.org/10.1007/s11657-020-0693-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-020-0693-z