Abstract
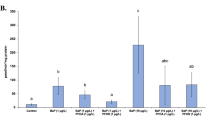
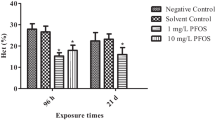
Perfluorinated organic compounds (PFOCs) are emerging persistent organic pollutants (POPs) widely distributed in the environment, wildlife and human. We studied the toxicology of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) on immunotoxicity and hepatotoxicity in primarily cultured hepatocytes of freshwater tilapia (Oreochromis niloticus). Cultured hepatocytes were exposed to PFOS or PFOA (0, 10, 20 and 30 mg/L) for 24, 48, 72, 96, 120 and 144 hours, respectively. Oral doses of these compounds that induce significantly detectable immunotoxicity and hepatotoxicity were employed in the study. In response to PFOS, the leukocytes, B cells, granulocytes, and macrophages among the isolation of intrahepatic immune cells (IHIC) from PFOS-treated tilapia produced significant levels of immune cells compared with that of the control group. The numbers of leukocytes, B cells, granulocytes, and macrophages for PFOS-treated tilapia increased with the incremental exposure concentration. Moreover, similar to the findings in PFOA toxicity effects, the erythropoietin levels in tilapia increased with the increase of the PFOS and PFOA concentrations. The lowest doses (10 mg/L) of PFOS exposure led to a marked inhibition in the hepatocyte viability in tilapia. Similarly, tilapia exposed to PFOA demonstrated a similar pattern, and a dose-dependent decrease in the hepatocyte viability was observed in the following treatment of PFOA. In the 72 h exposures, ethoxy-resorufin-O-deethylase (EROD) activity was significantly induced with the increase concentrations in tilapia liver (p<0.05). Tilapia showed a strong EROD induction in livers, and significantly difference in EROD activity was observed between control, PFOS and PFOA-exposed tilapias. The liver glycogen content showed that PFOS and PFOA exposure caused significant changes in the liver glycogen content, which depended on the duration of exposure. And it appeared that the decrease in blood glucose level during the acclimation was followed by significant increase in liver glycogen content in tilapia.
Similar content being viewed by others
References
Anna Lennquist, Malin C. Celander, and Lars Förlin (2008) Effects of medetomidine on hepatic EROD activity in three species of fish [J]. Ecotoxicology and Environmental Safety. 69, 74–79.
Andersen M.E., Butenhoff J.L., Chang S.C., Farrar D.G., Kennedy Jr G.L., Lau C., and Cockburn A. (2008) Perfluoroalkyl acids and related chemistries-toxicokinetics and modes of action [J]. Toxicol Sci. 102, 3–14.
Alexander J., Auounsson G.A., Benford D., Cockburn A., Cravedi J.P., and Dogliotti E. (2008) Perfluorooctanoic acid (PFOA) and their salts: Scientific opinion of the panel on contaminants in the food chain [J]. EFSA J. 653, 1–13.
Andersson T. and Part P. (1989) Benzowaxpyrene metabolism in isolated perfused rainbow trout gills [J]. Mar. Environ. Res. 28, 3–7.
Blom K.G., Qazi M.R., Matos J.B., Nelson B.D., DePierre J.W., and Abedi Valugerdi M. (2009) Isolation of murine intrahepatic immune cells employing a modified procedure for mechanical disruption and functional characterization of the B, T and natural killer T cells obtained [J]. Clin. Exp. Immunol. 155, 320–329.
Bucheli T.D. and Fent K. (1995) Induction of cytocrome P450 as a biomarker for environmental contaminate on in aquatic ecosystem [J]. Crit. Rev. Environ. Sci. Technol. 25, 201–268.
Hagenaars A., Knapen D., Meyer I.J., Van der Ven K., Hoff P., and De Coen W. (2008) Toxicity evaluation of perfluorooctane sulfonate (PFOS) in the liver of commoncarp (Cyprinus carpio) [J]. Aquatic Toxicology. 88, 155–163.
Liu Chunsheng, Yu Ke, Shi Xiongjie, Wang Jingxian, Lam Paul K.S., Rudolf S.S., and Zhou Bingsheng (2007) Induction of oxidative stress and apoptosis by PFOS and PFOA in primary cultured hepatocytes of freshwater tilapia (Oreochromis niloticus) [J]. Aquatic Toxicology. 82, 135–143.
DeWitt J.C., Copeland C.B., and Luebke R.W. (2009) Suppression of humoral immunity by perfluorooctanoic acid is independent of elevated serum corticosterone concentration in mice [J]. Toxicol Sci. 109, 106–112.
Dong Guanghui, Zhang Yinghua, Zheng Li, Liu Wei, Jin Yihe, and He Qincheng (2009) Chronic effects of perfluorooctanesulfonate exposure on immunotoxicity in adult male C57BL/6 mice [J]. Arch Toxicol. 83, 805–815.
DeVito M.J., Mauer W.E., Diliberto J.J., and Birnbaum L.S. (1993) Comparative ability of various PCBs, PCDFs, and TCDD to induce cytochrome P450 1A1 and 1A2 activity following 4 weeks of treatment [J]. Fundam. Appl. Toxicol. 20, 125–130.
Ericson I., Marti Cid R., Nadal M., Van Bavel B., Lindstrom G., and Domingo J.L. (2008) Human exposure to perfluorinated chemicals through the diet: intake of perfluorinated compounds in foods from the Catalan (Spain) market [J]. J. Agric. Food Chem. 56, 1787–1794.
Fromme H., Tittlemier S.A., Volkel W., Wilhelm M., and Twardella D. (2009) Perfluorinated compounds exposure assessment for the general population in Western countries [J]. Int. J. Hyg. Environ. Health. 212, 239–250.
Fried W. (2009) Erythropoietin and erythropoiesis [J]. Exp. Hematol. 37, 1007–1015.
Hivarininen A. and Nikkila A.E. (1962) Specific determination of blood glucose with O-toluidine [J]. Clin. Chem. Acta. 7, 140–143.
Kissa E. (2001) Fluorinated Surfactants and Repellents Surfactant Science Series [M]. pp.615–618. Marcel Dekker Inc., New York.
Key B.D., Howell R.D., and Criddle C.S. (1997) Fluorinated organics in the biosphere [J]. Environ. Sci. Technol. 31, 2445–2454.
Liu Chunsheng, Ke Yu, and Shi Xiongjie (2007) Induction of oxidative stress and apoptosis by PFOS and PFOA in primary cultured hepatocytes of freshwater tilapia (Oreochromis niloticus) [J]. Aquatic Toxicology. 82, 135–143.
Lehmler H.J. (2005) Synthesis of environmentally relevant fluorinated surfactants—a review [J]. Chemosphere. 58, 1471–1496.
Lau C., Anitole K., Hodes C., Lai D., and Pfahles Hutchens A. (2007) Seed J. Perfluoroalkyl acids: A review of monitoring and toxicological findings [J]. Toxicol Sci. 99, 366–394.
Livingstone D.R. (1994) Recent developments in marine invertebrate organic xenobiotic metabolism [J]. Toxicol. Ecotoxicol. News. 1, 8–94.
Martin J.W., Smithwick M.M., Braune B.M., Hoekstra P.F., Muir D.C.G., and Mabury S.A. (2004) Identification of long-chain perfluorinated acids in biota from the Canadian arctic [J]. Environ. Sci. Technol. 38, 373–380.
Mousumi Rahman Qazi and Mohammad Abedi R. (2010) Dietary exposure to perfluorooctanoate or perfluorooctane sulfonate induces hypertrophy in centrilobular hepatocytes and alters the hepatic immune status in mice [J]. International Immunopharmacology. 10, 1420–1427.
Miren Cajaraville P. and Maria Bebianno J. (2000) The use of biomarkers to assess the impact of pollution in coastal environments of the Iberian Peninsula: a practical approach [J]. The Science of the Total Environment. 247, 295–311.
OECD (2002) Hazard Assessment of Perfluorooctane Sulfonate (PFOS) and its Salts [S]. Organisation for Economic Co-operation and Development, Paris, France.
OECD (2005) Results of Survey on Production and Use of PFOS, PFAS and PFOA, Related Substances and Products/Mixtures Containing These Substances [S]. Organisation for Economic Co-operation and Development, Paris, France.
Olsen G.W., Burris J.M., Ehresman D.J., Froehlich J.W., Seacat A.M., and Butenhoff J.L. (2007) Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate and perfluorooctanoate in retired fluorochemical production workers [J]. Environ. Health Perspect. 115, 1298–1305.
Olivero Verbel J., Tao L., Johnson Restrepo B., Guette Fernandez J., Baldiris Avila R., O’byrne Hoyos I., and Kannan K. (2006) Perfluorooctanesulfonate and related fluorochemicals in biological samples from the north coast of Colombia [J]. Environ. Pollut. 142, 367–372.
Qazi M.R., Bogdanska J., Butenhoff J.L., Nelson B.D., DePierre J.W., and Abedi Valugerdi M. (2009) High-dose, short-term exposure of mice to perfluorooctanesulfonate (PFOS) or perfluorooctanoate (PFOA) affects the number of circulating neutrophils differently, but enhances the inflammatory responses of macrophages to lipopolysaccharide (LPS) in a similar fashion [J]. Toxicology. 262, 207–214.
Spliethoff H.M., Tao L., Shaver S.M., Aldous K.M., Pass K.A., and Kannan K. (2008) Use of newborn screening program blood spots for exposure assessment: Declining levels of perluorinated compounds in New York State infants [J]. Environ. Sci. Technol. 42, 5361–5367.
Seifter S., Dayton S., and Novic B. (1951) Muntwyler. The estimation of glycogen with the anthrone reagent [J]. Arch. Biochem. 25, 191–200.
Stegeman J.J., Brouwer M., Richard T.D.G., Förlin L., Fowler B.A., Sanders B.M., and Van Veld P.A. (1992) Molecular responses to environmental contamination: enzyme and protein systems as indicators of chemical exposure and effect. In Biomarkers: Biochemical, Physiological and Histological Markers of Anthropogenic Stress (eds. Huggett R.J., Kimerle R.A., and Bergman P.M.) [M]. pp.235–335. Lewis Publishers, Boca Raton, FL, USA.
Slavco Mitev, Suzana Dinevska Kovkarovska, and Biljana Miova (2005) Effect of the acclimation to high environmental temperature on the activity of hepatic glycogen phosphorylase, liver glycogen content and blood glucose level in rats [J]. Journal of Thermal Biology. 30, 563–568.
Li Tsengchiao, Liu Lilian, Chen Chienmin, and Ding Wanghsien (2006) Analysis of perfluorooctanesulfonate and related fluorochemicals in water and biological tissue samples by liquid chromatography-ion trap mass spectrometry [J]. J. Chromatogr. A. 1105, 119–126.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, Z., Liu, Y., Wu, D. et al. Immunotoxicity and hepatotoxicity of PFOS and PFOA in tilapia (Oreochromis niloticus). Chin. J. Geochem. 31, 424–430 (2012). https://doi.org/10.1007/s11631-012-0593-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-012-0593-z