Abstract
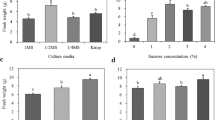
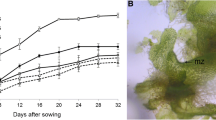
Propagation of gametophytes and sporophytes using mechanical fragmentation has been considered a suitable method for mass production of ferns. This study aimed to develop a practical propagation method for Lemmaphyllum microphyllum C. Presl, which is a fern of significant ornamental and medicinal value. Gametophytes were obtained through in vitro spore germination and used for propagation experiments. The gametophyte was mechanically fragmented using a scalpel into small fragments, which were then used to investigate gametophyte proliferation. In addition, the gametophyte was fragmented using a blender and then used to study sporophyte formation. Optimal proliferation conditions of the gametophyte were determined using Murashige and Skoog (MS) basal medium (double-, full-, half-, quarter-strength), Knop medium, and medium components (sucrose, nitrogen sources, activated charcoal), at various concentrations. The fresh weight of the gametophyte was 14-fold higher than that of gametophytes (300 mg) used as culture material, when cultured on double-strength MS. Moreover, 1 g of the gametophyte fragmented in 25 mL of distilled water formed more than 430 sporophytes in a soil mixture in an area of 7.5 cm2. The sporophytes were successfully cultivated in the greenhouse after acclimation. A large-scale production method for L. microphyllum that can be easily implemented in a fern production farm is outlined.



Similar content being viewed by others
References
Banks JA (1999) Gametophyte development in ferns. Annu Rev Plant Physiol Plant Mol Biol 50:163–186
Cooke RC (1979) Homogenization as an aid in tissue culture propagation of Platycerium and Davallia. HortSci 14:21–22
Dyer AF (1979) The culture of fern gametophytes for experimental investigation. In: Dyer AF (ed) The experimental biology of ferns. Academic, London, pp 254–305
Fernández H, Bertrand AM, Sánchez-Tamés R (1997) Plantlet regeneration in Asplenium nidus L. and Pteris ensiformis L. by homogenization of BA-treated rhizomes. Sci Hortic 68:243–247
Fernández H, Bertrand AM, Sánchez-Tamés R (1999) Biological and nutritional aspects involved in fern multiplication. Plant Cell Tiss Org Cult 56:211–214
Fernández H, Revilla MA (2003) In vitro culture of ornamental ferns. Plant Cell Tiss Org Cult 73:1–13
Gabriel y Galán JM, Migliaro G (2011) Comparative study on the gametophyte morphology and development of three paramo species of Jamesonia (Pteridaceae, Polypodiopsida). Nor J Bot 29:249–256
Hirsch AM (1975) The effect of sucrose on the differentiation of excised leaf tissue into either gametophytes or sporophytes. Plant Physiol 56:390–393
Ito M (1962) Studies on the differentiation of fern gametophytes I. regeneration of single cells isolated from cordate gametophytes of Pteris vittata. Bot Mag Tokyo 75:19–27
Jang BK, Cho JS, Kwon HJ, Lee CH (2019a) Optimal conditions for spore germination and gametophyte and sporophyte production in the autumn fern Dryopteris erythrosora. Hortic Environ Biotechnol 60:115–123
Jang BK, Cho JS, Lee CH (2019b) Propagation methods for gametophyte proliferation and sporophyte formation in silver cloak fern (Cheilanthes argentea). Hortic Environ Biotechnol 60:435–442
Jang BK, Cho JS, Park KT, Lee CH (2019c) A methodology for large-scale Athyrium sheareri gametophyte proliferation and sporophyte production using tissue culture. In Vitro Cell Dev Biol--Plant 55:519–526. https://doi.org/10.1007/s11627-019-09991-5
Kawano (2015) Pteridophytes as active components in gardening, agricultural and horticultural ecosystems in Japan. Adv Hortic Sci 29:41–47
Knauss JF (1976) A partial tissue culture method for pathogen-free propagation of selected ferns from spores. Proc Fla State Hort Soc 89:363–365
Knop W (1865) Quantitative Untersuchungen uber die Ernahrungsprozesse der Pflanzen. Landwirtsch Vers Stn 7:93–107
Kozai T (1991) Photoautotrophic micropropagation. In Vitro Cell Dev Biol--Plant 27:47–51
Kuriyama A, Kobayashi T, Hayashi S, Maeda M (2004) Medium composition for the production of sporophytes of the fern Adiantum capillus-veneris. J Jpn Soc Hortic Sci 73:580–582
Lee CS, Lee KH (2018) Pteridophytes of Korea: Lycophytes and ferns, 2nd edn. Geobook, Seoul
Maeda M, Ito M (1981) Isolation of protoplasts from fern prothallia and their regeneration to gametophytes. Bot Mag Tokyo 94:35–40
Melan MA, Whittier DP (1990) Effects of inorganic nitrogen sources on spore germination and gametophyte growth in Botrychium dissectum. Plant Cell Environ 13:477–482
Menéndez V, Arbesú R, Somer M, Revilla A, Fernández H (2010) From spore to sporophyte: how to proceed in vitro. In: Fernández H, Kumar A, Revilla A (eds) Working with ferns: issues and applications. Springer, New York, pp 97–110
Miller JH (1968) Fern gametophytes as experimental material. Bot Rev 34:361–440
Moran RC (2004) A natural history of ferns. Timber Press, Portland
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nelson PV (1991) Greenhouse operation and management. Prentice Hall, North Carolina
Pan MJ, van Staden J (1998) The use of charcoal in in-vitro culture – a review. Plant Growth Regul 26:155–163
Raghavan V (1989) Developmental biology of fern gametophytes. Cambridge University Press, UK
Renner GDR, Randi AM (2004) Effects of sucrose and irradiance on germination and early gametophyte growth of the endangered tree fern Dicksonia sellowiana Hook (Dicksoniaceae). Acta Bot Bras 18:375–380
Robertson RA (1993) Peat, horticulture and environment. Biodivers Conserv 2:541–547
Stamps RH, Evans MR (1999) Growth of Dracaena marginata and Spathiphyllum ‘Petite’ in Sphagnum peat and coconut coir dust-based growing media. J Environ Hortic 17:49–52
Thomas TD (2008) The role of activated charcoal in plant tissue culture. Biotechnol Adv 26:618–631
Waman AA, Pooja B, Sathyanarayana BN (2014) Not all sugars are sweet for banana multiplication. In vitro multiplication, rooting and acclimatization of banana as influenced by carbon source-concentration interactions. In Vitro Cell Dev Biol--Plant 50:552–560
Whittier DP (1990) Effects of nitrogen source on spore germination and gametophyte growth in Psilotum. Bot Gaz 151:50–53
Wu H, Liu XQ, Ji H, Chen LQ (2010) Effects of light, macronutrients, and sucrose on germination and development of the endangered fern Adiantum reniforme var. sinense (Adiantaceae). Sci Hortic 125:417–421
Zheng X, Xing F (2009) Ethnobotanical study on medicinal plants around Mt. Yinggeling, Hainan Island, China. J Ethnopharmacol 124:197–210
Acknowledgments
This study was supported by the “useful plant collection and development of mass propagation protocol for the establishment of the foundation of convergence platform using forest plants (KNA1-2-25, 16-3)” project funded by Korea National Arboretum.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Editor: Masaru Nakano
Rights and permissions
About this article
Cite this article
Jang, B.K., Cho, J.S., Park, K. et al. Practical methodology for gametophyte proliferation and sporophyte production in green penny fern (Lemmaphyllum microphyllum C. Presl) using mechanical fragmentation. In Vitro Cell.Dev.Biol.-Plant 56, 318–324 (2020). https://doi.org/10.1007/s11627-020-10055-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-020-10055-2