Summary
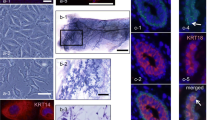
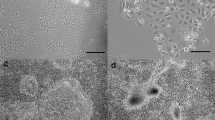
EpH4 is a nontumorigenic cell line derived from spontaneously immortalized mouse mammary gland epithelial cells (Fialka et al., 1996). When grown in collagen gels, EpH4 cells give rise to different types of structures, e.g., solid cords or branching tubes. By removing and subsequently dissociating single three-dimensional colonies of defined morphology, we have isolated six clonal subpopulations of EpH4 cells which display distinct morphogenetic properties in collagen gel cultures. Thus, cells from the H1B clone form branching cords devoid of a central lumen, K3A3 cells from cords enclosing small multifocal lumina, and J3B1 cells form large cavitary structures containing a wide lumen. I3G2 cells form either cords or tubes, depending on the type of serum added to the culture medium. Finally, when grown in serum-free medium, Be1a cells form spherical cysts, whereas Be4a cells form long, extensively branched tubes. In additional assays of morphogenesis, i.e., cell sandwiching between two collagen gels or culture on a thick layer of Matrigel (a laminin-rich extracellular matrix), all clones form epithelial-cell-lined cavitary structures, except H1B cells which are unable to generate lumina under these conditions. The EpH4 sublines we have isolated provide an in vitro system for studying the mechanisms responsible for lumen formation and branching morphogenesis, as well as for identifying the factors which subvert these developmental processes during mammary carcinogenesis.
Similar content being viewed by others
References
Barcellos-Hoff, M. H.; Aggeler, J.; Ram, T. G., et al. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 105:223–235; 1989.
Berdichevsky, F.; Alford, D.; D’Souza, B., et al. Branching morphogenesis of human mammary epithelial cells in collagen gels. J. Cell Sci. 107:3557–3568; 1994.
Chambard, M.; Gabrion, J.; Mauchamp, J. Influence of collagen gel on the orientation of epithelial cell polarity: follicle formation from isolated thyroid cells and from preformed monolayers. J. Cell Biol. 91:157–166; 1981.
Daniel, C. W.; Silberstein, G. B. Postnatal development of the rodent mammary gland. In: Neville, M. C.; Daniel, C. W., ed. The mammary gland. Development, regulation and function. New York: Plenum Press; 1987:3–36.
Danielson, K. G.; Oborn, C. J.; Durban, E. M., et al. Epithelial mouse mammary cell line exhibiting normal morphogenesis in vivo and functional differentiation in vitro. Proc. Natl. Acad. Sci. USA 81:3756–3760; 1984.
Darcy, K. M.; Black, J. D.; Hahm, H. A., et al. Mammary organoids from immature virgin rats undergo ductal and alveolar morphogenesis when grown within a reconstituted basement membrane. Exp. Cell Res. 196:49–65; 1991.
Fialka, I.; Schwartz, H.; Reichmann, E., et al. The estrogen-dependent c-JunER protein causes a reversible loss of mammary epithelial cell polarity involving a destabilization of adherens junctions. J. Cell Biol. 132:1115–1132; 1996.
Gumbiner, B. M. Epithelial morphogenesis. Cell 69:385–387; 1992.
Hall, H. G.; Farson, D. A.; Bissell, M. J. Lumen formation by epithelial cell lines in response to collagen overlay: a morphogenetic model in culture. Proc. Natl. Acad. Sci. USA 79:4672–4676; 1982.
Hurley, W. L.; Blatchford, D. R.; Hendry, K. A. K., et al. Extracellular matrix and mouse mammary cell function: comparison of substrata in culture. In Vitro Cell. Dev. Biol. 30A:529–538; 1994.
Kanazawa, T.; Hosik, H. L. Transformed growth phenotype of mouse mammary epithelium in primary culture induced by specific fetal mesenchymes. J. Cell. Physiol. 153:381–391; 1992.
Keely, P. J.; Fong, A. M.; Zutter, M. M., et al. Alteration of collagen-dependent adhesion, motility, and morphogenesis by the expression of antisense α2 integrin mRNA in mammary cells. J. Cell Sci. 108:595–607; 1995.
Kleinman, H. K.; McGarvey, M. L.; Hassel, J. R., et al. Basement membrane complexes with biological activity. Biochemistry 25:312–318; 1986.
López-Barahona, M.; Fialka, I.; Gonzalez-Sancho, J. M., et al. Thyroid hormone regulates stromelysin expression, protease secretion and the morphogenetic potential of normal polarized mammary epithelial cells. EMBO J. 14:1145–1155; 1995.
Montesano, R.; Orci, L.; Vassalli, P. In vitro rapid organization of endothelial cells into capillary-like networks is promoted by collagen matrices. J. Cell Biol. 97:1648–1652; 1983.
Montesano, R.; Schaller, G.; Orci, L. Induction of epithelial tubular morphogenesis in vitro by fibroblast-derived soluble factors. Cell 66:697–711; 1991.
Oft, M.; Peli, J.; Rudaz, C., et al. TGF-β1 and Ha-Ras collaborate in modulating the phenotype plasticity and invasiveness of epithelial tumor cells. Genes Devel. 10:2462–2477; 1996.
Ormerod, E. J.; Rudland, P. S. Mammary gland morphogenesis in vitro: formation of branched tubules in collagen gels by a cloned rat mammary cell line. Dev. Biol. 91:360–375; 1982.
Petersen, O. W.; Rønnov-Jensen, L.; Howlett, A. R., et al. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. USA 89:9064–9068; 1993.
Reichmann, E.; Ball, R.; Groner, B., et al. New mammary epithelial and fibroblastic cell clones in coculture form structures competent to differentiate functionally. J. Cell Biol. 108:1127–1138; 1989.
Reichmann, E.; Schwartz, H.; Deiner, E. M., et al. Activation of an inducible c-FosER fusion protein causes loss of epithelial polarity and triggers epithelial-fibroblastoid cell conversion. Cell 71:1103–1116; 1992.
Schwimmer, R.; Ojakian, G. K. The α2β1 integrin regulates collagen-mediated MDCK epithelial membrane remodeling and tubule formation. J. Cell Sci. 108:2487–2498; 1995.
Soriano, J. V.; Orci, L.; Montesano, R. TGF-β1 induces morphogenesis of branching cords by cloned mammary epithelial cells at subpicomolar concentrations. Biochem. Biophys. Res. Commun. 220:879–885; 1996.
Soriano, J. V.; Pepper, M. S.; Nakamura, T., et al. Hepatocyte growth factor stimulates extensive development of branching duct-like structures by cloned mammary gland epithelial cells. J. Cell Sci. 108:413–430; 1995.
Soule, H. D.; Maloney, T. M.; Wolman, S. R., et al. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 50:6075–6086; 1990.
Stahl, S.; Weitzman, S.; Jones, J. C. R. The role of laminin-5 and its receptor in mammary epithelial cell branching morphogenesis. J. Cell Sci. 110:55–63; 1997.
Vernon, R. B.; Angello, J.-C.; Iruela-Arispe, M. L., et al. Reorganization of basement membrane matrices by cellular traction promotes the formation of cellular networks in vitro. Lab. Invest. 66:536–547; 1992.
Wohlwend, A.; Vassalli, J.-D.; Belin, D., et al. LLC-PK1 cells: cloning of phenotypically stable subpopulations. Am. J. Physiol. 250:C682-C687; 1986.
Yang, J.; Guzman, R.; Richards, J., et al. Primary culture of human mammary epithelial cells embedded in collagen gels. J. Natl. Cancer Inst. 65:337–343; 1980.
Yang, Y.; Spitzer, E.; Meyer, D., et al. Sequential requirement of hepatocyte growth factor and neuregulin in the morphogenesis and differentiation of the mammary gland. J. Cell Biol. 131:215–226; 1995.
Zuk, A.; Matlin, K. S. Apical β1 integrin in polarized MDCK cells mediates tubulocyst formation in response to type I collagen overlay. J. Cell Sci. 109:1875–1889; 1996.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Montesano, R., Soriano, J.V., Fialka, I. et al. Isolation of EpH4 mammary epithelial cell subpopulations which differ in their morphogenetic properties. In Vitro Cell.Dev.Biol.-Animal 34, 468–477 (1998). https://doi.org/10.1007/s11626-998-0080-3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11626-998-0080-3