Abstract
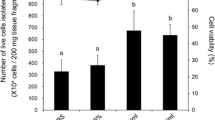
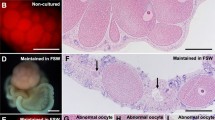
In the present work, primary cell cultures from ovaries of the edible sea urchin Paracentrotus lividus were developed in order to provide a simple and versatile experimental tool for researches in echinoderm reproductive biology. Ovary cell phenotypes were identified and characterized by different microscopic techniques. Although cell cultures could be produced from ovaries at all stages of maturation, the cells appeared healthier and viable, displaying a higher survival rate, when ovaries at early stages of gametogenesis were used. In terms of culture medium, ovarian cells were successfully cultured in modified Leibovitz-15 medium, whereas poor results were obtained in minimum essential medium Eagle and medium 199. Different substrates were tested, but ovarian cells completely adhered only on poly-L-lysine. To improve in vitro conditions and stimulate cell proliferation, different serum-supplements were tested. Fetal calf serum and an originally developed pluteus extract were detrimental to cell survival, apparently accelerating processes of cell death. In contrast, cells cultured with sea urchin egg extract appeared larger and healthier, displaying an increased longevity that allowed maintaining them for up to 1 month. Overall, our study provides new experimental bases and procedures for producing successfully long-term primary cell cultures from sea urchin ovaries offering a good potential to study echinoid oogenesis in a controlled system and to investigate different aspects of echinoderm endocrinology and reproductive biology.





Similar content being viewed by others
References
Barbaglio A.; Sugni M.; Di Benedetto C.; Bonasoro F.; Schnell S.; Lavado R.; Porte C.; Candia Carnevali D. M. Gametogenesis correlated with steroid levels during the gonadal cycle of the sea urchin Paracentrotus lividus (Echinodermata: Echinoidea). Comp. Biochem. Physiol. A 147(2): 466–474; 2007.
Brooks J. M.; Wessel G. M. The major yolk protein in sea urchins is a transferrin-like, iron binding protein. Dev. Biol. 245(1): 1–12; 2002.
Byrne M. Annual reproductive cycles of the commercial sea-urchin Paracentrotus lividus from an exposed intertidal and a sheltered subtidal habitat on the west-coast of Ireland. Mar. Biol. 104(2): 275–289; 1990.
Candia Carnevali D. M. Regeneration in Echinoderms: repair, regrowth, cloning. Invertebr. Surviv. J. 3: 64–76; 2006.
Cao A.; Mercado L.; Ramos-Martinez J. I.; Barcia R. Primary cultures of hemocytes from Mytilus galloprovincialis Lmk.: expression of IL-2Rα subunit. Aquaculture 216(1–4): 1–8; 2003.
Cervello M.; Matranga V. Evidence of a precursor-product relationship between vitellogenin and toposome, a glycoprotein complex mediating cell adhesion. Cell Differ. Dev. 26(1): 67–76; 1989.
Chatlynne L. G. A histochemical study of oogenesis in the sea urchin, Strongylocentrotus purpuratus. Biol. Bull. 136(2): 167–184; 1969.
Chia FS, Bickell, L. R. (1983) Echinodermata. In: Adiyod KGAaRG (ed) Reproductive biology of invertebrates. spermatogenesis and sperm function, vol 2. K.G. Adiyodi & R.G. Adiyodi New York, pp 545–620
de Caralt S.; Uriz M. J.; Wijffels R. H. Cell culture from sponges: pluripotency and immortality. Trends Biotechnol. 25(10): 467–471; 2007.
Fenaux L. Maturation des gonads et cycle saisonnier des larves chez A. lixula, P. lividus et P. microtuberculatus (Echinides) a’ Villefranche-sur-mer. Vie Milieu 19: 1–52; 1968.
Giga Y.; Ikaia A. Purification of the most abundant protein in the coelomic fluid of a sea urchin which immunologically cross reacts with 23S glycoprotein in the sea urchin eggs. J. Biochem. 98(1): 19–26; 1985.
Houk M. S.; Hinegardner R. T. The formation and early differentiation of sea urchin gonads. Biol. Bull. 159(2): 280–294; 1980.
Kanatani H.; Nagahama Y. Echinodermata. In: Adiyodi K. G. A. R. G. (ed) Reproductive biology of invertebrates. spermatogenesis, ooogenesis, oviposition, and absorption. 1st ed. John Wiley & Sons, New York, pp 545–620; 1983.
Leoni V, Fernandez C, Johnson M, Ferrat L, Pergent-Martini C (2001) Preliminary study on spawning periods in the sea urchin Paracentrotus lividus from lagoon and marine environments. Echinoderm Research:277–280
McCarron E.; Burnell G.; Kerry J.; Mouzakitis G. An experimental assessment on the effects of photoperiod treatments on the somatic and gonadal growth of the juvenile European purple sea urchin Paracentrotus lividus. Aquac. Res. 41(7): 1072–1081; 2010.
Mita M. Relaxin-like gonad-stimulating substance in an echinoderm, the starfish: A novel relaxin system in reproduction of invertebrates. Gen. Comp. Endocr. 181: 241–245; 2013.
Moss C.; Beesley P. W.; Thorndyke M. C.; Bollner T. Preliminary observations on ascidian and echinoderm neurons and neural explants in vitro. Tissue Cell 30(5): 517–524; 1998.
Mulford A. L.; Austin B. Development of primary cell cultures from Nephrops norvegicus. Method Cell Sci. 19(4): 269–275; 1998.
Noll H.; Alcedo J.; Daube M.; Frei E.; Schiltz E.; Hunt J.; Humphries T.; Matranga V.; Hochstrasser M.; Aebersold R.; Lee H.; Noll M. The toposome, essential for sea urchin cell adhesion and development, is a modified iron-less calcium-binding transferrin. Dev. Biol. 310(1): 54–70; 2007.
Odintsova N. A.; Dolmatov I. Y.; Mashanov V. S. Regenerating holothurian tissues as a source of cells for long-term cell cultures. Mar. Biol. 146(5): 915–921; 2005.
Odintsova N. A.; Ermak A. V.; Tsal L. G. Substrate selection for long-term cultivation of marine invertebrate cells. Comp. Biochem. Physiol. A 107(4): 613–619; 1994.
Poccia D. In vitro differentiation of male germ line cells from sea urchin testis. J. Exp. Zool. 246(1): 57–64; 1988.
Rinkevich B. Cell cultures from marine invertebrates: obstacles, new approaches and recent improvements. In: R. Osinga JTJGB, Wijffels RH (eds) Progress in Industrial Microbiology, vol 35. Elsevier, pp 133–153; 1999
Rinkevich B. Cell cultures from marine invertebrates: new insights for capturing endless stemness. Mar. Biotechnol. 13(3): 345–354; 2011.
Sharlaimova N. S.; Pinaev G. P.; Petukhova O. A. Comparative analysis of behavior and proliferative activity in culture of cells of coelomic fluid and of cells of various tissues of the sea star Asterias rubens L. isolated from normal and injured animals. Cell Tissue Biol. 4(3): 280–288; 2010.
Shashikumar A.; Desai P. V. Development of cell line from the testicular tissues of crab Scylla serrata. Cytotechnology 63(5): 473–480; 2011.
Shpigel M.; McBride S. C.; Marciano S.; Lupatsch I. The effect of photoperiod and temperature on the reproduction of European sea urchin Paracentrotus lividus. Aquaculture 232(1–4): 343–355; 2004.
Spirlet C.; Grosjean P.; Jangoux M. Reproductive cycle of the echinoid Paracentrotus lividus: analysis by means of the maturity index. Invertebr. Reprod. Dev. 34(1): 69–81; 1998.
Spirlet C.; Grosjean P.; Jangoux M. Optimization of gonad growth by manipulation of temperature and photoperiod in cultivated sea urchins, Paracentrotus lividus (Lamarck) (Echinodermata). Aquaculture 185(1–2): 85–99; 2000.
Takahashi N.; Kanatani H. Effect of 17β-estradiol on growth of oocytes in cultured ovarian fragments of the starfish, Asterina pectinifera. Dev. Growth Differ. 23(6): 565–569; 1981.
Unuma T.; Konishi K.; Kiyomoto M.; Matranga V.; Yamano K.; Ohta H.; Yokota Y. The major yolk protein is synthesized in the digestive tract and secreted into the body cavities in sea urchin larvae. Mol. Reprod. Dev. 76(2): 142–150; 2009.
Unuma T.; Suzuki T.; Kurokawa T.; Yamamoto T.; Akiyama T. A protein identical to the yolk protein is stored in the testis in male red sea urchin, Pseudocentrotus depressus. Biol. Bull. 194(1): 92–97; 1998.
Walker C. W.; McGinn N. A.; Harrington L. M.; Lesser M. P. Reproduction in sea urchins. In: Lawrence J. (ed) The Biology and Ecology of Edible Sea Urchins. El Sevier Press, Amsterdam; 2000.
Walton A.; Smith V. J. Primary culture of the hyaline hemocytes from marine decapods. Fish Shellfish Immunol. 9(3): 181–194; 1999.
Yakovlev K. V.; Battulin N. R.; Serov O. L.; Odintsova N. A. Isolation of oogonia from ovaries of the sea urchin Strongylocentrotus nudus. Cell Tissue Res. 342(3): 479–490; 2010.
Acknowledgment
We are grateful to the “Area Marina” of Bergeggi (SV) and Portofino (GE) for giving their permission to collect experimental animals.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Rights and permissions
About this article
Cite this article
Mercurio, S., Di Benedetto, C., Sugni, M. et al. Primary cell cultures from sea urchin ovaries: a new experimental tool. In Vitro Cell.Dev.Biol.-Animal 50, 139–145 (2014). https://doi.org/10.1007/s11626-013-9686-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-013-9686-1