Abstract
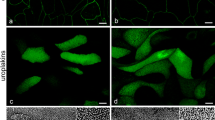
When the urothelial barrier, i.e., the blood−urine barrier, is injured, rapid resealing of the injury is crucial for the normal functioning of the organism. In order to investigate the mechanisms required for rapid resealing of the barrier, we established in vitro models of hyperplastic and normoplastic urothelia. We found that hyperplastic urothelia achieve significantly higher transepithelial resistance (TER) than normoplastic urothelia. However, the expression of cell junctional (claudin-8, occludin, E-cadherin) and differentiation-related proteins (cytokeratin 20 and uroplakins) is weaker in hyperplastic urothelia. Further investigation of cell differentiation status at the ultrastructural level confirmed that superficial urothelial cells (UCs) in hyperplastic urothelial models achieve a lower differentiation stage than superficial UCs in normoplastic urothelial models. With the establishment of such in vitro models and the aid of TER measurements, flow cytometry, molecular and ultrastructural analysis, we here provide unequivocal evidence that the specific cell-cycle distribution and, consequently, the number of cell layers have a significant influence on the barrier function of urothelia. We demonstrate the importance of hyperplasia for the rapid restoration of the urothelial barrier and the maintenance of high TER until the UCs reach a highly differentiated stage and restoration of the urothelial barrier after injury is complete. The information that this approach provides is unique and we expect that further exploitation of hyperplastic and normoplastic urothelial models in future studies may advance our understanding of blood−urine barrier development and functionality.





Similar content being viewed by others
References
da Rocha MS, Nascimento MG, Cardoso AP, de Lima PL, Zelandi EA, de Camargo JL, de Oliveira ML (2010) Cytotoxicity and regenerative proliferation as the mode of action for diuron-induced urothelial carcinogenesis in the rat. Toxicol Sci 113:37–44
Farsund T (1976) Cell kinetics of mouse urinary bladder epithelium. II. Changes in proliferation and nuclear DNA content during necrosis regeneration, and hyperplasia caused by a single dose of cyclophosphamide. Virchows Arch B Cell Pathol 21:279–298
Fromter E, Diamond J (1972) Route of passive ion permeation in epithelia. Nat New Biol 235:9–13
Fukushima S, Arai M, Cohen S, Jacobs JB, Friedell GH (1981) Scanning electron microscopy of cyclophosphamide-induced hyperplasia of the rat urinary bladder. Lab Invest 44:89–96
Hicks RM (1965) The fine structure of the transitional epithelium of rat ureter. J Cell Biol 26:25–48
Hicks RM (1975) The mammalian urinary bladder: an accomodating organ. Biol Rev 50:215–246
Hicks RM, Wakefield JJ (1976) Membrane changes during urothelial hyperplasia and neoplasia. Cancer Res 36:2502–2507
Hu P, Meyers S, Liang FX, Deng FM, Kachar B, Zeidel ML, Sun TT (2002) Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol 283:F1200–F1207
Jezernik K, Romih R, Mannherz HG, Koprivec D (2003) Immunohistochemical detection of apoptosis, proliferation and inducible nitric oxide synthase in rat urothelium damaged by cyclophosphamide treatment. Cell Biol Int 27:863–869
Koss LG, Lavin P (1970) Effects of a single dose of cyclophosphamide on various organs in the rat. II. Response of urinary bladder epithelium according to strain and sex. J Natl Cancer Inst 44:1195–2000
Kreft ME, Romih R, Sterle M (2002) Antigenic and ultrastructural markers associated with urothelial cytodifferentiation in primary explant outgrowths of mouse bladder. Cell Biol Int 26:63–74
Kreft ME, Sterle M, Veranic P, Jezernik K (2005) Urothelial injuries and the early wound healing response: tight junctions and urothelial cytodifferentiation. Histochem Cell Biol 123:529–539
Kreft ME, Sterle M, Jezernik K (2006) Distribution of junction- and differentiation-related proteins in urothelial cells at the leading edge of primary explant outgrowths. Histochem Cell Biol 125:475–485
Kreft ME, Di Giandomenico D, Beznoussenko GV, Resnik N, Mironov AA, Jezernik K (2010) Golgi apparatus fragmentation as a mechanism responsible for uniform delivery of uroplakins to the apical plasma membrane of uroepithelial cells. Biol Cell 102:593–607
Kreplak L, Wang H, Aebi U, Kong XP (2007) Atomic force microscopy of mammalian urothelial surface. J Mol Biol 374:365–373
Kunze E, Köhnecke B, Engelhardt W, Steinröder H, Brock N, Pohl J (1984) Effect of the uroprotector sodium 2-mercaptoethane sulfonate (Mesna) on the proliferation of the bladder urothelium in the rat after administration of cyclophosphamide. Urol Int 39:61–67
Lavelle J, Meyers S, Ramage R, Bastacky S, Doty D, Apodaca G, Zeidel ML (2002) Bladder permeability barrier: recovery from selective injury of surface epithelial cells. J Physiol Renal Physiol 283:F242–F253
Lewis SA, Diamond JM (1976) Na+ transport by rabbit urinary bladder, a tight epithelium. J Memb Biol 28:1–40
Locher GW, Cooper EH (1970) Repair of rat urinary bladder epithelium following injury by cyclophosphamide. Invest Urol 8:116–123
Martin BF (1972) Cell replacement and differentiation in transitional epithelium: a histological and autoradiographic study of the guinea-pig bladder and ureter. J Anat 112:433–455
Min G, Zhou G, Schapira M, Sun TT, Kong XP (2003) Structural basis of urothelial permeability barrier function as revealed by Cryo-EM studies of the 16 nm uroplakin particle. J Cell Sci 116:4087–4094
Molon-Noblot S, Boussiquet-Leroux C, Owen RA, Irisarri E, Duran-Cavagna G, Peter CP, Duprat P (1992) Rat urinary bladder hyperplasia induced by oral administration of carbonic anhydrase inhibitors. Toxicol Pathol 20:93–102
Negrete HO, Lavelle JP, Berg J, Lewis SA, Zeidel ML (1996) Permeability properties of the intact mammalian bladder epithelium. Am J Physiol 271:F886–F894
Parsons CL, Boychuk D, Jones S, Hurst R, Callahan H (1990) Bladder surface glycosaminoglycans: an epithelial permeability barrier. J Urol 143:139–142
Romih R, Jezernik K, Masera A (1998) Uroplakins and cytokeratins in the regenerating rat urothelium after sodium saccharin treatment. Histochem Cell Biol 109:263–269
Romih R, Veranic P, Jezernik K (1999) Actin filaments during terminal differentiation of urothelial cells in the rat urinary bladder. Histochem Cell Biol 112:375–380
Romih R, Korosec P, de Mello W Jr, Jezernik K (2005) Differentiation of epithelial cells in the urinary tract. Cell Tissue Res 320:259–268
Truschel ST, Ruiz WG, Shulman T, Pilewski J, Sun TT, Zeidel ML, Apodaca G (1999) Primary uroepithelial cultures. A model system to analyze umbrella cell barrier function. J Biol Chem 274:15020–15029
Veranic P, Jezernik K (2000) The response of junctional complexes to induced desquamation in mouse bladder urothelium. Biol Cell 92:105–113
Veranic P, Erman A, Kerec-Kos M, Bogataj M, Mrhar A, Jezernik K (2009) Rapid differentiation of superficial urothelial cells after chitosan-induced desquamation. Histochem Cell Biol 131:129–139
Acknowledgments
We thank Prof. Dr. Kristijan Jezernik and Prof. Dr. Rok Romih (University of Ljubljana, Faculty of Medicine, Institute of Cell Biology) for their continuous support. The authors thank Prof. Dr. Tung-Tien Sun (New York University Medical School) for the generous gift of uroplakin antibodies. The study was supported by the Slovenian Ministry of Higher Education, Science and Technology (Grant No P3-0108).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Višnjar, T., Kocbek, P. & Kreft, M.E. Hyperplasia as a mechanism for rapid resealing urothelial injuries and maintaining high transepithelial resistance. Histochem Cell Biol 137, 177–186 (2012). https://doi.org/10.1007/s00418-011-0893-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-011-0893-0