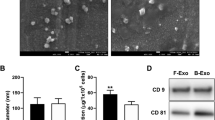
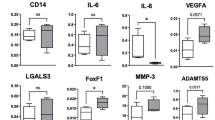
Abstract
In vitro studies using chondrocyte cell cultures have increased our understanding of cartilage physiology and the altered chondrocytic cell phenotype in joint diseases. Beside the use of primary cells isolated from cartilage specimens of donors, immortalized chondrocyte cell lines such as C-28/I2 and T/C-28a2 have facilitated reproducible and standardized experiments. Although carbohydrate structures appear of significance for cartilage function, the contribution of the chondrocyte glycocalyx to matrix assembly and alterations of the chondrocyte phenotype is poorly understood. Therefore, the present study aimed to evaluate the glycoprofile of primary human chondrocytes as well as of C-28/I2 and T/C-28a2 cells in culture. First, the chondrocytic phenotype of primary and immortalized cells was assessed using real-time reverse transcriptase polymerase chain reaction, immunofluorescence, and glycosaminoglycans staining. Then, a panel of lectins was selected to probe for a range of oligosaccharide sequences determining specific products of the O-glycosylation and N-glycosylation pathways. We found that differences in the molecular phenotype between primary chondrocytes and the immortalized chondrocyte cell models C-28/I2 and T/C-28a2 are reflected in the glycoprofile of the cells. In this regard, the glycocalyx of immortalized chondrocytes was characterized by reduced levels of high-mannose type and sialic acid-capped N-glycans as well as increased fucosylated O-glycosylation products. In summary, the present report emphasizes the glycophenotype as an integral part of the chondrocyte phenotype and points at a significant role of the glycophenotype in chondrocyte differentiation.





Similar content being viewed by others
References
Aigner, T.; Stove, J. Collagens—major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair. Adv. Drug Deliv. Rev. 55: 1569–1593; 2003 doi:10.1016/j.addr.2003.08.009.
Akama, T. O.; Nakagawa, H.; Wong, N. K.; Sutton-Smith, M.; Dell, A.; Morris, H. R.; Nakayama, J.; Nishimura, S. I.; Pai, A.; Moremen, K. W.; Marth, J. D.; Fukuda, M. N. Essential and mutually compensatory roles of α-mannosidase II and α-mannosidase IIx in N-glycan processing in vivo in mice. PNAS 103: 8983–8988; 2006 doi:10.1073/pnas.0603248103.
Baldus, S. E.; Thiele, J.; Park, Y. O.; Hanisch, F. G.; Bara, J.; Fischer, R. Characterization of the binding specificity of Anguilla anguilla agglutinin (AAA) in comparison to Ulex europaeus agglutinin I (UEA-I). Glycoconjugate J 13: 585–590; 1996 doi:10.1007/BF00731446.
Bernard, B. A.; DeLuca, L. M.; Hassell, J. R.; Yamada, K. M.; Olden, K. Retinoic acid alters the proportion of high mannose to complex type oligosaccharides on fibronectin secreted by cultured chondrocytes. J. Biol. Chem. 259: 5310–5315; 1984.
Carlsson, H. E.; Lonngren, J.; Goldstein, I. J.; Christner, J. E.; Jourdian, G. W. The interaction of wheat germ agglutinin with keratan from cornea and nasal cartilage. FEBS Lett. 62: 38–40; 1976 doi:10.1016/0014-5793(76)80011-7.
Cohen, M.; Klein, E.; Geiger, B.; Addadi, L. Organization and adhesive properties of the hyaluronan pericellular coat of chondrocytes and epithelial cells. Biophys. J. 85: 1996–2005; 2003 doi:10.1016/S0006-3495(03)74627-X.
Finger, F.; Schoerle, C.; Zien, A.; Gebhard, P.; Goldring, M. B.; Aigner, T. Molecular phenotyping of human chondrocyte cell lines T/C-28a2, T/C-28a4 and C-28/I2. Arthritis Rheum. 48: 3395–3403; 2003 doi:10.1002/art.11341.
Goldring, M. B.; Birkhead, J. R.; Suen, L. F.; Yamin, R.; Mizuno, S.; Glowacki, J.; Arbiser, J. L.; Apperley, J. F. Interleukin-1β-modulated gene expression in immortalized human chondrocytes. J. Clin. Invest. 94: 2307–2316; 1994 doi:10.1172/JCI117595.
Helenius, A.; Aebi, M. Intracellular functions of N-linked glycans. Science 23: 2364–2369; 2001 doi:10.1126/science.291.5512.2364.
Hoedt-Schmidt, S.; Scheid, A.; Kalbhen, D. A. Histomorphological and lectin-histochemical confirmation of the antidegenerative effect of diclofenac in experimental osteoarthrosis. Arzneimittelforschung 39: 1212–1219; 1989.
Kiernan, J. A. Dyes and other colorants in microtechnique and biomedical research. Color. Technol. 122: 1–21; 2006 doi:10.1111/j.1478-4408.2006.00009.x.
Knudson, C. B.; Knudson, W. Cartilage Proteoglycans. Semin. Cell Dev. Biol. 12: 69–78; 2001 doi:10.1006/scdb.2000.0243.
Lee, J. Y.; Spicer, A. P. Hyaluronan: a multifunctional, megaDalton, stealth molecule. Curr. Opin. Cell Biol. 12: 581–586; 2000 doi:10.1016/S0955-0674(00)00135-6.
Liener, I. E.; Sharon, N.; Goldstein, I. J. The lectins, properties, functions, and applications in biology and medicine. Academic, Orlando1986.
Martin, I.; Jakob, M.; Schaefer, D.; Dick, W.; Spagnoli, G.; Heberer, M. Quantitative analysis of gene expression in articular cartilage from normal and osteoarthritic joints. Osteoarthr. Cartilage 9: 112–118; 2001 doi:10.1053/joca.2000.0366.
Oliver, B. L.; Cronin, C. G.; Zhang-Benoit, Y.; Goldring, M. B.; Tanzer, M. L. Divergent stress responses to IL-1b, nitric oxide, and tunicamycin by chondrocytes. J. Cell Physiol. 204: 45–50; 2005 doi:10.1002/jcp.20261.
Piana, C.; Guell, I.; Gerbes, S.; Gerdes, R.; Mills, C.; Samitier, J.; Samitier, J.; Wirth, M.; Gabor, F. Influence of surface modification on vitality and differentiation of Caco-2 cells. Differentiation 75: 308–317; 2007 doi:10.1111/j.1432-0436.2006.00141.x.
Stokes, D. G.; Liu, G.; Coimbra, I. B.; Piera-Velazques, S.; Crowl, R. M.; Jimenez, S. A. Assessment of the gene expression profile of differentiated and dedifferentiated human fetal chondrocytes by microarray analysis. Arthritis Rheum. 46: 402–419; 2002 doi:10.1002/art.10106.
Takagaki, K.; Nakamura, T.; Izumi, J.; Saitoh, H.; Endo, M. Characterization of hydrolysis and transglycosylation by testicular hyaluronidase using ion-spray mass spectrometry. Biochemistry 33: 6503–6507; 1994 doi:10.1021/bi00187a017.
Toda, N.; Doi, A.; Jimbo, A.; Matsumoto, I.; Seno, N. Interaction of sulphated glycosaminoglycans with lectins. J. Biol. Chem. 256: 5345–5349; 1981 doi:10.1186/1471-2199-8-13.
Toegel, S.; Harrer, N.; Plattner, V. E.; Unger, F. M.; Viernstein, H.; Goldring, M. B.; Gabor, F.; Wirth, M. Lectin binding studies on C-28/I2 and T/C-28a2 chondrocytes provide a basis for new tissue engineering and drug delivery perspectives in cartilage research. J. Control Release 117: 121–129; 2007a.
Toegel, S.; Huang, W.; Piana, C.; Unger, F. M.; Wirth, M.; Goldring, M. B.; Gabor, F.; Viernstein, H. Selection of reliable reference genes for qPCR studies on chondroprotective action. BMC Mol. Biol. 8: 13; 2007b doi:10.1016/j.jconrel.2006.10.004.
Toegel, S.; Wu, S. Q.; Unger, F. M.; Wirth, M.; Goldring, M. B.; Gabor, F.; Viernstein, H. Comparison between chondroprotective effects of glucosamine, curcumin, and diacerein in IL-1β stimulated C-28/I2 chondrocytes. Osteoarthr Cartilage 16: 1205–1212; 2008 doi:10.1016/j.joca.2008.01.013.
Von der Mark, K.; Gauss, V.; Von der Mark, H.; Muller, P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 267: 531–532; 1977 doi:10.1038/267531a0.
Yamamoto, K.; Tsuji, T.; Matsumoto, I.; Osawa, T. Structural requirements for the binding of oligosaccharides and glycopeptides to immobilized wheat germ agglutinin. Biochemistry 20: 5894–5899; 1981 doi:10.1021/bi00523a037.
Yang, X.; Lehotay, M.; Anastassiades, T.; Harrison, M.; Brockhausen, I. The effect of TNF-α on glycosylation pathways in bovine synoviocytes. Biochem. Cell Biol. 82: 559–568; 2004 doi:10.1139/o04-058.
Yang, X.; Yip, J.; Anastassiades, T.; Harrison, M.; Brockhausen, I. The action of TNFα and TGFβ include specific alterations of the glycosylation of bovine and human chondrocytes. Biochim. Biophys. Acta 1773: 264–272; 2007 doi:10.1016/j.bbamcr.2006.09.022.
Acknowledgments
Stefan Toegel gratefully acknowledges Stratagene for the Stratagene Research Grant 2005. Part of the work was supported by the Integrated Project CellProm (NMP4-CT-2004-500039) granted from the sixth framework program of the European Community. Dr. Goldring’s research is supported by grant R01-AG022021.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Denry Sato
Rights and permissions
About this article
Cite this article
Toegel, S., Plattner, V.E., Wu, S.Q. et al. Lectin binding patterns reflect the phenotypic status of in vitro chondrocyte models. In Vitro Cell.Dev.Biol.-Animal 45, 351–360 (2009). https://doi.org/10.1007/s11626-009-9186-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-009-9186-5