Abstract
Objective
We examined the clinical utility of supplementing type 2 diabetes mellitus (DM) risk counseling with DM genetic test results and counseling.
Research Design and Methods
In this randomized controlled trial, non-diabetic overweight/obese veteran outpatients aged 21 to 65 years received DM risk estimates for lifetime risk, family history, and fasting plasma glucose, followed by either genetic test results (CR+G; N = 303) or control eye disease counseling (CR+EYE; N = 298). All participants received brief lifestyle counseling encouraging weight loss to reduce the risk of DM.
Results
The mean age was 54 years, 53% of participants were black, and 80% were men. There was no difference between arms in weight (estimated mean difference between CR+G vs. CR+EYE at 3 months = 0.2 kg, 95% CI: −0.3 to 0.7; at 6 months = 0.4 kg, 95 % CI: −0.3 to 1.1), insulin resistance, perceived risk, or physical activity at 3 or 6 months. Calorie and fat intake were lower in the CR+G arm at 3 months (p’s ≤ 0.05) but not at 6 months (p’s > 0.20).
Conclusions
Providing patients with genetic test results was not more effective in changing patient behavior to reduce the risk of DM compared to conventional risk counseling.
Trial registration: ClinicalTrials.gov NCT01060540 http://clinicaltrials.gov/show/NCT01060540
Similar content being viewed by others
INTRODUCTION
Type 2 diabetes mellitus (DM) is a major cause of disability, reduced quality of life, and early mortality,1–3 and its prevalence has increased dramatically in parallel with the rising rates of obesity.3,4 However, DM can be delayed or prevented via lifestyle modification and medication.5 In the Diabetes Prevention Program (DPP), weight loss was a stronger predictor of DM prevention than dietary fat reduction, increased physical activity, or treatment with metformin.6,7 Despite the effectiveness of weight loss for reducing the risk of DM, motivating patients to initiate weight loss behaviors can be challenging.
Clinicians frequently inform patients of their disease risk in order to encourage weight loss measures. Indeed, disease risk communication is a key component of behavior theories.8,9 Common DM risk factors include demographic characteristics, body mass index (BMI), family history, and blood glucose. While genetic markers have been shown to modestly increase the risk of DM risk,10 patients with these markers can reduce risk through lifestyle modification.11,12 The clinical utility of these markers (i.e., the extent to which they improve patient outcomes) has not been established. We conducted a randomized controlled trial (RCT) to examine whether supplementing conventional DM risk counseling with genetic test counseling and results would affect short-term clinical and behavioral outcomes associated with DM prevention.
METHODS
Design
Details on the study design and protocol were previously reported.13 In this two-arm RCT, participants attended a risk counseling session with a genetic counselor that involved conventional DM risk counseling plus either a) genetic counseling and test results (CR+G) or b) control eye disease counseling (CR+EYE). The primary outcome was weight at 3 months post-enrollment. We chose weight because it is proximal to the development of DM. We assumed that, in the absence of intensive intervention, any impact of genetic risk counseling would be observed in the short term. Secondary outcomes included weight at 6 months; insulin resistance, physical activity, and dietary intake at 3 and 6 months; and perceived risk immediately following risk counseling and at 3 and 6 months. Ethics approval was obtained from the local institutional review board.
Setting, Population, and Recruitment
Participants were recruited from primary care panels at the Durham Veterans Affairs Medical Center (VAMC) and two satellite clinics. Enrollment began in January 2011; follow-up was complete in March 2013. Non-diabetic patients were eligible if they were aged 21 to 65 years, had baseline BMI ≥ 27 kg/m2, and were not actively losing weight. Recruitment occurred via letter and telephone call to patients meeting these criteria who had upcoming primary care appointments or who self-referred in response to flyers.
Baseline Visit
Participants provided written informed consent and were asked not to obtain genetic testing from outside resources while enrolled. Age, race, sex, weight, and height were collected by a research assistant (RA) to calculate lifetime risk for DM. Family history of DM was obtained for first- and second-degree relatives. Blood was drawn for fasting plasma glucose (FPG), fasting insulin, and, depending on arm assignment, genetic testing. Perceived risk and level of physical activity14 were obtained orally by the RA. A food frequency questionnaire15 was completed by participants at home due to its length.
Randomization
Participants were randomized in blocks within strata defined by weight status (BMI < 35 kg/m2 vs. ≥ 35 kg/m2) and family history of DM (unknown/low vs. moderate/high). The project coordinator entered BMI, family history, and baseline FPG values into the study database. Once eligibility was confirmed, the randomization scheme was generated using SAS (SAS Institute, Cary, NC, USA). The project coordinator placed a sheet with arm assignment in an opaque envelope to be opened by the genetic counselor during the counseling session; the genetic counselor and participants were blinded until this point. RA’s were blinded to arm assignment throughout the study.
DM Risk Counseling
In both arms, conventional risk counseling was delivered by one of two genetic counselors, who provided information on definition, prevalence, negative outcomes, and risk factors for DM. Participants received personalized estimates for a) lifetime DM risk based on age, race, sex, and BMI16, b) family history-based DM risk17, and c) FPG-based DM risk. Participants in the CR+G arm had blood analyzed for three DM-related genes (Rs7903146C>T on TCF7L2, Rs1801282C>G on PPARγ, and Rs5219T>C on KCNJ11).13 These single-nucleotide polymorphisms (SNPs) were among the most studied and validated in mixed-race populations at study conception.18 Although additional SNPs were discovered after startup, we were unable to test them. These additional SNPs have not substantially increased clinical validity.19 Genetic testing was performed at the Duke University Clinical Molecular Diagnostics Laboratory.
In adherence to guidelines for enhanced risk communication,20 risk was communicated with plain language and visually represented with vertical bar graphs using traffic light colors, as these were perceived as effective by our target population.13 For each risk factor, a participant’s level of risk was assigned to one of three categories (low = green, moderate = yellow, high = red), with data on the various risk factors based on different statistics, time frames, and reference groups. This method of risk categorization is consistent with the notion that people focus on gist rather than numerical precision of risk information.21
After patients were presented with risk information based on demographics/BMI, family history, and FPG, the genetic counselor opened the opaque envelope containing the randomization assignment, and participants in the CR+G arm received more extensive information on genetics and its role in DM. They were informed that DM genetic markers represent increased risk rather than a definitive determination of the eventual presence of DM. CR+G participants received personalized genetic test results using a color-coded vertical bar graph. Patients were informed that we had tested three of the best-validated genetic markers, that risk levels could differ if more markers were tested, and that lifestyle modification could prevent or delay DM onset even if genetic results indicated increased risk.
Participants in the CR+EYE arm received education on age-related macular degeneration, cataracts, and glaucoma, as there is little overlap between these recommended preventive strategies and DM prevention strategies. Although DM is a risk factor for eye disease, the genetic counselor did not emphasize this risk, such that the control counseling would be unlikely to motivate participants with regard to preventing DM. The control eye disease counseling was matched in duration to the genetic counseling.13 CR+EYE participants did not receive genetic testing.
Next, all participants received brief lifestyle counseling encouraging weight loss. Participants were urged to reduce calories, portions, and refined carbohydrate intake, to increase intake of non-starchy vegetables and lean proteins, and to engage in moderate physical activity and/or walking at least 30 min per day, five days per week.22 Metformin use was not addressed. To mimic a plausible scenario of implementing genetic counseling and testing in primary care, participants were not subsequently enrolled in an intensive lifestyle intervention. All were, however, advised of a weight loss program freely available to VA patients (MOVE!).23 Participants were provided with a summary of the risk information and informational pamphlets about DM, diet, physical activity, and, in the CR+G arm, genetic information.13
Fidelity to the risk counseling protocol was maximized by development of a slide set, standardized training for genetic counselors, audio recording of > 50% of the counseling sessions, and review of a random subset of the recorded sessions and direct observation by the first author.
Outcome Measures
Clinical and health behavior outcomes were assessed at baseline and at 3 and 6 months, with 3 months as the primary endpoint. Assessment of all outcomes except perceived lifetime risk of DM were conducted by an RA blinded to arm assignment; perceived risk was collected by the genetic counselor at the end of the risk counseling session and by a blinded RA at 3 and 6 months. Participants received $25 for each assessment visit.
Weight was measured on a standardized digital scale, with participants wearing light clothing and shoes removed. Blood for FPG and insulin was obtained after a 12-hour fast. Improvements in insulin action were determined by calculating insulin resistance (HOMA2-IR) using the updated homeostasis model assessment (HOMA) calculator (http://www.dtu.ox.ac.uk/homacalculator/). Perceived lifetime risk was evaluated with a single item (“What are your chances of getting type 2 diabetes in your lifetime”) on a scale anchored by “definitely will not get diabetes”1 and “definitely will get diabetes” 7. Dietary intake was assessed with the Block Brief 2000 Food Frequency Questionnaire (FFQ),15 with a 3-month recall period. We analyzed calorie, carbohydrate, protein, and fat intake, as these were emphasized in our counseling protocol. Daily physical activity was evaluated using the long version of the International Physical Activity Questionnaire (IPAQ)14; we analyzed moderate physical activity and walking, which were emphasized in our counseling protocol.
Statistical Analyses
Power and sample size calculations assumed a null hypothesis of no between-arm difference in 3-month weight, and used methods for analysis of covariance in randomized trials.24 We assumed a standard deviation of approximately 24.8 kg at 3 months, a correlation of 0.90 between baseline and 3-month weights, and a 3-month attrition rate of 10%.25 With an α = 0.05 (two-sided), a sample size of 300 per arm yielded 80% power to detect a clinically meaningful 2.7 kg difference in weight between arms.26
Following intention-to-treat standards, participants were analyzed in their assigned arms, regardless of risk counseling attendance.27 Statistical analyses were performed using SAS for Windows (Version 9.2; SAS Institute, Cary, NC, USA). For primary analyses, we fit repeated-measures linear mixed models (LMM) with an unstructured covariance model.28 Primary predictors included indicators for the 3- and 6-month follow-up times and interactions between treatment arm and follow-up time, following a constrained longitudinal data analysis (cLDA) approach in which baseline measurements were assumed to be equal between arms.29 All available patient data were used. The final models included stratification variables for weight status and family history of DM.
We followed a similar modeling strategy for the continuous secondary outcomes. The perceived risk LMM included additional indicator variables for the post-counseling follow-up time and the interaction of treatment by post-counseling follow-up. Intake of calories, saturated fat, monounsaturated fat, and polyunsaturated fat were log-transformed. For physical activity variables, we fit a generalized LMM using a negative binomial distribution with a log link function, since the distribution of these variables followed a Poisson-type process.30 In preplanned exploratory analyses, we examined whether the intervention effect on weight differed by family history risk level or genetic counselor and, among participants in the CR+G arm, genetic risk level (see online supplement).
RESULTS
Participants
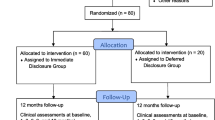
Recruitment letters were mailed to 3621 patients meeting initial eligibility criteria, and 83 additional patients self-referred (Fig. 1). Of 3342 patients for whom eligibility was assessed via telephone, 675 were scheduled for baseline interviews. Of those, 47 declined enrollment at the baseline visit prior to consent. Of 628 patients who consented to participate, 27 were excluded due to low weight or high FPG values. Thus, 601 patients were randomized (303 to CR+G, 298 to CR+EYE), of whom 534 attended the risk counseling session. Outcome assessment visits were attended by 506 participants at 3 months (84%) and 472 participants at 6 months (79%).
The mean age was 54 years, and most participants were black, male, and had a post-high school education (Table 1). Nearly one-third had a BMI ≥ 35 kg/m2, and more than half had moderate or high family history-based DM risk. Over 60% of participants had high lifetime DM risk, whereas over 80% had low DM risk based on baseline FPG levels. In the CR+G arm, 24% were at high genetic risk, 37% were at moderate risk, and 38% were at low risk.
Primary and Secondary Outcomes
Estimated mean weight did not differ between arms at 3 months [(CR+G)−(CR+EYE) = 0.2 kg, 95 % CI: −0.3, 0.7; p = 0.44] or at 6 months (mean difference = 0.4 kg, 95 % CI: −0.3, 1.1; p = 0.27), nor did insulin resistance (HOMA2-IR) (p = 0.19 and 0.12 at 3 and 6 months, respectively; Table 2). Perceived lifetime risk did not differ between arms at any time point (all p > 0.38). Daily calorie intake was lower in the CR+G arm than the CR+EYE arm at 3 months (p = 0.05), but there was no difference between arms at 6 months (p = 0.20). The percentage of calories from carbohydrates, protein, fat, and saturated fat intake did not differ between arms at 3 or 6 months (all p ≥ 0.07). Monounsaturated fat and polyunsaturated fat were lower in the CR+G than the CR+EYE arm at 3 months (all p ≤ 0.04) but not at 6 months (all p ≥ 0.26). There were no between-arm differences in the estimated duration of moderate-intensity physical activity or walking at 3 or 6 months (all p > 0.22; Table 3).
Post hoc analyses showed no differential treatment effect on weight by family history risk level at 3 (p = 0.99 for interaction) or 6 months (p = 0.98 for interaction). There was no differential treatment effect on weight by genetic counselor at 3 (p = 0.37 for interaction) or 6 months (p = 0.21 for interaction). Among CR+G participants, there was no difference in weight by genetic risk level at 3 or 6 months (all p = 0.42 and 0.36 for interactions, respectively).
Intervention Cost
Intervention cost in the CR+G arm was estimated individually for each genetic counselor because they differed in the average amount of time spent conducting intervention activities (118 vs. 82 min) and in salary. Given an estimated $7.00 per session in overhead costs, genetic testing cost of $125, and blood draw cost of $3.00 per participant, the total intervention cost was $207.03 for the first genetic counselor and $178.78 for the second.
DISCUSSION
Although intensive lifestyle interventions are the gold standard for weight loss,31 a single brief intervention can motivate some patients to change their behavior,32 and reflects the reality of most primary care settings. Therefore, it is important to determine whether brief interventions provided in primary care can be augmented by strategies such as incorporation of genetic counseling and testing. Communicating genetic risk for DM had a positive but small effect on dietary intake at 3 months that was not sustained at 6 months and did not translate to clinically meaningful weight loss or improvements in insulin sensitivity at either time point. The largely null effect occurred despite delivery of the intervention by a genetic counselor specifically trained to communicate genetic risk and to encourage risk-reduction behavior and with the addition of brief behavioral goal-setting.
Genetic information is likely to affect risk-reduction behavior if it is perceived to be the only, or strongest, risk factor.35 We informed participants that genetic information was one of several risk factors, that the magnitude of various risk factors could differ across individuals, and that risk could be reduced by behavior change even in the presence of increased genetic risk. Reactions to risk information tend to be heterogeneous and unpredictable, however, underscoring the importance of our study.
Our findings are consistent with a study in which 108 overweight patients were randomized to either receive or not receive genetic testing prior to initiating a 12-week lifestyle intervention.33 In comparisons of higher- and lower-risk patients to no-test controls, there were no differences in risk perception, motivation, confidence, program attendance, or weight loss. Our study had a larger sample size, was powered to detect a clinically significant difference in 3-month weight (instead of program attendance or readiness to change), and included patients of all risk levels (instead of excluding moderate-risk participants). Despite differences in the primary research question and methods, findings from these two studies converged to suggest that communication of genetic test results using currently available methods is unlikely to produce a meaningful effect in DM prevention.
One limitation of our study was that we tested only three of at least 65 genetic markers that have been identified. Although scores comprising combinations of genetic markers may have increased prediction in some groups, the range of odds ratios remained less than 2.0, lower than the risk conferred by family history.36,37 Indeed, some may question the value of evaluating the clinical utility of genetic information when the data on clinical validity remains questionable.19 The answer is that patient perceptions are often not aligned with objective data, and genetic markers could therefore have great clinical utility even if not substantial clinical validity.34 Given the time required to conduct and disseminate the findings from trials such as ours, it is important to investigate clinical utility even as researchers seek genetic markers that increase clinical validity. As additional markers and risk scores are validated, the underlying algorithm to classify risk levels can be updated to accommodate this new information.
Another limitation was that health behaviors were self-reported and thus were subject to recall bias and social desirability. A third limitation was that we were unable to examine the incidence of DM given the study duration and sample size. A fourth limitation was the lack of adequate power to conduct subgroup analyses. We powered on the main effect of arm assignment rather than an interaction with genetic risk level (in the CR+G arm), because of the uncertainty about how genetic risk level might affect outcomes: people who learn that they are at high genetic risk may become motivated or fatalistic, increasing or decreasing adherence to health behaviors, respectively. A fifth limitation was that the rate of missing data was greater than the 10% rate we assumed for our power calculation, and a greater amount of data was missing for the FFQ than for other measures because it was completed at home. Missingness was equal between arms, and our modeling approach yielded unbiased results when missing outcomes were related to either observed covariates or responses.38 Finally, our study was conducted in a single VAMC, which may limit generalizability.
One strength of our study was the randomized prospective design, which allowed us to examine the incremental effectiveness of genetic counseling and testing beyond conventional risk factors. DM is also an excellent prototype disease for this issue because a) patients wish to avoid developing what can be a debilitating disease, b) much is known about the environmental and genetic contributors to its development, and c) evidence underscores behavior change as the best way to prevent its development.
Given the multiple and increasing demands in primary care, absent compelling evidence of clinical utility, providers are unlikely to add genetic counseling and testing to their repertoire. On the basis of our study and others, it is premature to offer genetic testing for DM in primary care. Future research may identify patient subgroups for whom DM genetic counseling and testing have a clinically meaningful impact on outcomes or more powerful ways of delivering genetic risk information to engage patients in preventive behaviors. Until such subgroups or methods are identified, patients are unlikely to realize health benefits from DM genetic testing.
References
Adriaanse MC, Snoek FJ, Dekker JM, et al. Perceived risk for Type 2 diabetes in participants in a stepwise population-screening programme. Diabet Med. 2003;20:210–215.
Wee H-L, Cheung Y-B, Li S-C, Fong K-Y, Thumboo J. The impact of diabetes mellitus and other chronic medical conditions on health-related Quality of Life: Is the whole greater than the sum of its parts? Health Qual Life Outcome. 2005;3:2.
Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140:945–950.
Centers for Disease Control and Prevention: National Diabetes Fact Sheet, 2011, Fact Sheet. Atlanta, GA: Department of Health and Human Services - Center for Disease Control and Prevention, 2011.
Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–2107.
Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686.
Janz NK, Becker MH. The Health Belief Model: A decade later. Health Educ Q. 1984;11:1–47.
Weinstein N. What does it mean to understand a risk? Evaluation risk comprehension. J Natl Cancer Inst Monogr. 1999;25:15–12.
Morris AP, Voight BF, Teslovich TM, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981–990.
Hivert MF, Jablonski KA, Perreault L, et al. Updated genetic score based on 34 confirmed type 2 diabetes Loci is associated with diabetes incidence and regression to normoglycemia in the diabetes prevention program. Diabetes. 2011;60:1340–1348.
Florez JC, Jablonski KA, Bayley N, et al. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med. 2006;355:241–250.
Voils CI, Coffman CJ, Edelman D, et al. Examining the impact of genetic testing for type 2 diabetes on health behaviors: Study protocol for a randomized controlled trial. Trials. 2012;13:121.
Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395.
Block G: Block Brief 2000 Questionnaire, 2000.
Narayan KM, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care. 2007;30:1562–1566.
Hariri S, Yoon P, Qureshi N, et al. Family history of type 2 diabetes: A population-based screening tool for prevention? Genet Med. 2006;8:102–108.
Weedon MN, McCarthy MI, Hitman G, et al. Combining information from common type 2 diabetes risk polymorphisms improves disease prediction. PLoS Med. 2006;3:e374.
Lyssenko V, Laakso M. Genetic screening for the risk of type 2 diabetes: Worthless or valuable? Diabetes Care. 2013;36:S120–S126.
Fagerlin A, Zikmund-Fisher B, Ubel PA. Helping patients decide: Ten steps to better risk communication. J Natl Cancer Inst. 2011;103:1–8.
Nelson W, Reyna VF, Fagerlin A, Lipkus I, Peters E. Clinical implications of numeracy: Theory and practice. Ann Behav Med. 2008;35:261–274.
US Department of Health and Human Services: 2008 Physical Activity Guidelines for Americans, 2008.
Kahwati LC, Lance TX, Jones KR, Kinsinger LS. RE-AIM evaluation of the Veterans Health Administration’s MOVE! weight management program. Transl Behav Med. 2011;1:551–560.
Borm G, Fransen J, Lemmens W. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60:1234–1238.
Yancy W Jr, Westman E, McDuffie J, et al. A randomized trial of a low-carbohydrate, ketogenic diet versus orlistat plus a low-fat diet for weight loss. Arch Intern Med. 2010;170:136–145.
Lindstrom J, Louheranta A, Mannelin M, et al. The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003;26:3230–3236.
Group IEEW. ICH harmonised tripartite guideline-Statistical principles for clinical trials. Stat Med. 1999;18:1905–1942.
Verbeke G, Molenbergh G. Linear mixed models for longitudinal data. New York: Springer-Verlag; 2000.
Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. Hoboken, NJ: Wiley; 2004.
Diggle PJ, Heagerty P, Liang K, Zeger SL. Analysis of longitudinal data. New York: Oxford University Press; 2002.
Jensen MD, Ryan DH, Apovian CM, et al. AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2013;2014(129):S102–138.
Lewis A, Jolly K, Adab P, et al. A brief intervention for weight management in primary care: study protocol for a randomized controlled trial. Trials. 2013;14:393.
Grant RW, O’Brien KE, Waxler JL, et al. Personalized genetic risk counseling to motivate diabetes prevention: A randomized trial. Diabetes Care. 2013;36:13–19.
Vorderstrasse AA, Cho A, Voils CI, Orlando LA, Ginsburg GS. Clinical utility of genetic testing in primary care: The example of type 2 diabetes. Per Med. 2013;10:549–563.
Marteau TM, Weinman J. Self-regulation and the behavioural response to DNA risk information: A theoretical analysis and framework for future research. Soc Sci Med. 2006;62:1360–1368.
Vassy JL, Shrader P, Jonsson A, et al. Association between parental history of diabetes and type 2 diabetes genetic risk scores in the PPP-Botnia and Framingham Offspring Studies. Diabetes Res Clin Pract. 2011;93:e76–79.
de Miguel-Yanes JM, Shrader P, Pencina MJ, et al. Genetic risk reclassification for type 2 diabetes by age below or above 50 years using 40 type 2 diabetes risk single nucleotide polymorphisms. Diabetes Care. 2011;34:121–125.
Rubin DB. Inference and missing data. Biometrika. 1976;63:581–592.
Acknowledgments
This research was supported by a grant from the Department of Veterans Affairs (DVA) Health Services Research and Development (HSR&D) service (IIR 09–039). Dr. Maciejewski was supported by a Research Career Scientist award from DVA HSR&D (RCS 10–391). Results of this study were presented at The Obesity Society in November of 2013. Drs. Voils and Coffman and Ms. Grubber had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. We express heartfelt gratitude to Tamika Brown, BS, and Cherisa Williams, MBA, for their assistance with data collection; to Jennifer Hoff, MStat, for development of the study database; to Karen Nicely, MS, for delivery of genetic counseling; and to Michael Datto, MD, PhD, and Francoise Blanpain for providing genetic testing. The views expressed in this article are those of the authors and do not necessarily represent the views of the DVA.
Conflict of interest statement
Dr. Maciejewski has received consulting fees from Daiichi Sankyo Co. Ltd. and the Research Data Assistance Center (ResDAC) at the University of Minnesota, and owns Amgen stock due to his spouse’s employment. The other authors declare that they have no competing interests.
Authors’ Contributions
CIV obtained funding, participated in the design and coordination, assisted with developing the counseling protocol, oversaw recruitment, and drafted the manuscript. CJC participated in the design, assisted in refining the risk communication graphs, performed the power analysis, designed the analytic plan, performed statistical analyses, and assisted in drafting the manuscript. JMG assisted in developing the study database, wrote the code for generating the risk communication graphs, performed statistical analyses, and assisted in drafting the manuscript. DE participated in the design, assisted in refining the risk communication graphs, developed procedures for the rapid enrollment of our large study population, and provided critical revisions to the manuscript for important intellectual content. AS created the genetic counseling protocol and provided critical revisions to the manuscript. MLM conducted cost analyses, assisted in drafting the manuscript, and provided critical revisions for important intellectual content. JB assisted with development and implementation of the recruitment protocol and outcomes assessment, and managed the research assistants and genetic counselors. AC made substantial contributions to the conception and design of the project. GG helped develop the study consent process and method for delivery of genetic testing results and provided critical revisions to the manuscript. WSY obtained funding for the project, assisted in drafting the manuscript, and revised it critically for important intellectual content. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 50 kb)
Rights and permissions
About this article
Cite this article
Voils, C.I., Coffman, C.J., Grubber, J.M. et al. Does Type 2 Diabetes Genetic Testing and Counseling Reduce Modifiable Risk Factors? A Randomized Controlled Trial of Veterans. J GEN INTERN MED 30, 1591–1598 (2015). https://doi.org/10.1007/s11606-015-3315-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-015-3315-5