Abstract
Background
The receptor for advanced glycation end-products (RAGE) is implicated in pancreatic tumorigenesis. Activating Kras mutations and p16 inactivation are genetic abnormalities most commonly detected as pancreatic ductal epithelium progresses from intraepithelial neoplasia (PanIN) to adenocarcinoma (PDAC).
Objective
The aim of this study was to evaluate the effect of RAGE (or AGER) deletion on the development of PanIN and PDAC in conditional Kras G12D mice.
Materials and Methods
Pdx1-Cre; LSL-Kras G12D/+ mice were crossed with RAGE −/− mice to generate Pdx1-Cre; LSL-Kras G12D/+ ; RAGE −/− mice. Pdx1-Cre; LSL-Kras G12D/+; p16 Ink4a−/− mice were crossed with RAGE −/− mice to generate Pdx1-Cre; LSL-Kras G12D/+; p16 Ink4a−/−; RAGE −/− mice. Pancreatic ducts were scored and compared to the relevant RAGE +/+ controls.
Results
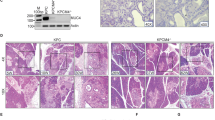
At 16 weeks of age, Pdx1-Cre; LSL-Kras G12D/+; RAGE −/− mice had significantly fewer high-grade PanIN lesions than Pdx1-Cre; LSL-Kras G12D/+; RAGE +/+ controls. At 12 weeks of age, none of the Pdx1-Cre; LSL-Kras G12D/+; p16 Ink4a−/−; RAGE −/− mice had PDAC compared to a 45.5% incidence of PDAC in Pdx1-Cre; LSL-Kras G12D/+; p16 Ink4a−/−; RAGE +/+ controls. Finally, Pdx1-Cre; LSL-Kras G12D/+; p16 Ink4a−/−; RAGE −/− mice also displayed markedly longer median survival.
Conclusion
Loss of RAGE function inhibited the development of PanIN and progression to PDAC and significantly prolonged survival in these mouse models. Further work is needed to target the ligand–RAGE axis for possible early intervention and prophylaxis in patients at risk for developing pancreatic cancer.






Similar content being viewed by others
References
Jemal A, Siegel R., Xu J, Ward E. Cancer statistics, 2010. CA cancer J Clin 2010;60:277–300.
Maitra A, Fukushima N, Takaori K, Hruban RH. Precurors to invasive pancreatic cancer. Adv Anat Pathol 2005;12:81–91.
Hruban RH, Goggins M, Parsons J, Kern SE. Genetic progression in the pancreatic ducts. Am J Pathol 2000;156:1821–1825.
Hingorani SR, Petrcoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Wright CVE, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer cell 2003;4:437–450.
Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, Feng B, Brennan C, Weissleder R, Mahmood U, Hanahan D, Redston MS, Chin L, DePinho RA. Both p16Ink4a and the p19Arf-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. PNAS 2006;103:5947–5952.
Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes & Dev 2003;17:3112–3126.
Bardessy N, Morgan J, Sinha M, Signoretti S, Srivastava S, Loda M, Merlino G, DePinho RA. Obligate roles for p16Ink4a and p19Arf-p53 in the suppression of murine pancreatic neoplasia. Mol Cell Biol 2002;22:635–643.
Han SH, Kim YH, Mook-Jung I. RAGE: The beneficial and deleterious effects by diverse mechanisms of actions. Mol Cells 2011;31:91–97.
Kang R, Tang D, Schapiro NE, Livesey KM, Farkas A, Loughran P, Bierhaus A, Lotze MT, Zeh HJ. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis promoting pancreatic tumor cell survival. Cell Death Differ 2010;17:666–676.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008;454:436–444.
Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 2009;457:102–107.
Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 2001;108:949–955.
Logsdon CD, Fuentes MK, Huang EH, Arumugam T. RAGE and RAGE ligands in cancer. Curr Mol Med 2007;7:777–789.
Riehl A, Németh J, Angel P, Hess J. The receptor RAGE: bridging inflammation and cancer. Cell Comm Sig 2007;7:12.
Rojas A, Figueroa H, Morales E. Fueling inflammation at tumor microenvironment: the role of multiligand/RAGE axis. Carcinogenesis 2010;31:334–341.
Ramasamy R, Yan SF, Schmidt AM. RAGE: therapeutic target and biomarker of the inflammatory response—the evidence mounts. J Leukoc Biol 2009;86:505–512.
Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 2010;28:367–388.
Takada M, Koizumi T, Toyama H, Suzuki Y, Kuroda Y. Differential expression of RAGE in human pancreatic carcinoma cells. Hepatogastroenterology 2001;48:1577–1578.
Takada M, Hirata K, Ajiki T, Suzuki Y, Kuroda Y. Expression of receptor for advanced glycation end products (RAGE) and MMP-9 in human pancreatic cancer cells. Hepatogastroenterology 2004;51:928–930.
Krechler T, Jachymova M, Mestek O, Zak A, Zima T, Kalousova M. Soluble receptor for advanced glycation end-products (sRAGE) and polymorphisms or RAGE and glyoxalase I genes in patients with pancreas cancer. Clin Biochem 2010;43:882–886.
Whiteman HJ, Weeks ME, Dowen SE, Barry S, Timms JF, Lemoine NR, Crnogorac-Jurcevic T. The role of S100P in the invasion of pancreatic cancer cells is mediated through cytoskeletal changes and regulation of cathepsin D. Cancer Res 2007;67:8633–8642.
DiNorcia J, Moroziewicz DN, Ippagunta N, Lee MK, Foster M, Rotterdam HZ, Bao F, Zou YS, Yan SF, Emond J, Schmidt AM, Allendorf JD. RAGE signaling significantly impacts tumorigenesis and hepatic tumor growth in murine models of colorectal carcinoma. J Gastrointest Surg 2010;14:1680–90.
Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes & Dev 2001;15:3243–3248.
Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, Aguirre AJ, Wu EA, Horner JW, DePinho RA. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 2001;413:86–91.
Wendt TM, Tanji N, Guo J, Kislinger TR, Qu W, Lu Y, Bucciarelli LG, Rong LL, Moser B, Markowitz GS, Stein G, Bierhaus. A, Liliensiek B, Arnold B, Nawroth PP, Stern DM, D'Agati VD, Schmidt AM. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol 2003;162:1123–1137.
Sakaguchi T, Yan SF, Yan SD, Belov D, Rong LL, Sousa M, Andrassy M, Marso SP, Duda S, Arnold B, Liliensiek B, Nawroth PP, Stern DM, Schmidt AM, Naka Y. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest 2003;111:959–972.
Funahashi H, Satake M, Dawson D, Huynh NA, Reber HA, Hines OJ, Eibl G. Delayed progression of pancreatic intraepithelial neoplasia in a conditional KrasG12D mouse model by a selective cyclooxygenase-2 inhibitor. Cancer Res 2007;67:7068–7071.
Hruban RH, Rustgi AK, Brentnall TA, Tempero MA, Wright CV, Tuveson DA. Pancreatic cancer in mice and man: the Penn Workshop 2004. Cancer Res 2006;66:14–7.
Hruban RH, Adsay NV, Albores-Saavedra J, Anver MR, Biankin AV, Boivin GP, Furth EE, Furukawa T, Klein A, Klimstra DS, Kloppel G, Lauwers GY, Longnecker DS, Luttges J, Maitra A, Offerhaus GJ, Perez-Gallego L, Redston M, Tuveson DA. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res 2006;66:95–106.
Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hor O, Ogawa S, Stern DM, Schmidt AM. Blockade of amphoterin/RAGE signaling suppresses tumor growth and metastases. Nature 2001;405:354–360.
Shi G, Shu L, Sun Y, Bettencourt R, Damsz B, Hruban RH, Konieczny SF. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology 2009;136:1328–1378.
Lee KE, Bar-Sagi D. Oncogenic KRas suppresses inflammation-associated senescence of pancreatic ductal cells. Cancer Cell 2010;18:448–458.
Fendrich V, Schneider R, Maitra A, Jacobsen ID, Opfermann T, Bartsch DK. Detection of precursor lesions of pancreatic adenocarcinoma in PET-CT in a genetically engineered mouse model of pancreatic cancer. Neoplasia 2011;13:180–186.
Rowley M, Ohashi A, Mondal G, Mills L, Yang L, Zhang L, Sundsbak R, Shapiro V, Muders MH, Smyrk T, Couch FJ. Inactivation of Brca2 promotes Trp53-associated but inhibits KrasG12D-dependent pancreatic cancer development in mice. Gastroenterology 2011;140:1303–1313.
Hill R, Calvopina JH, Kim C, Wang Y, Dawson DW, Donahue TR, Dry S, Wu H. PTEN loss accelerates KrasG12D-induced pancreatic cancer development. Cancer Res 2010;70:2114–7124.
Mohammed A, Janakiram NB, Li Q, Madka V, Ely M, Lightfoot S, Crawford H, Steele VE, Rao CV. The epidermal growth factor receptor inhibitor Gefitinib prevents the progression of pancreatic lesions to carcinoma in a conditional LSL-KrasG12D/+ transgenic mouse model. Cancer Prev Res 2010;3:1417–1426.
Arumugam T, Simeone DM, Van Golen K, Logsdon CD. S100P promotes pancreatic cancer growth, survival, and invasion. Clin Cancer Res 2005;11:5356–5364.
Arumugam T, Ramachandran V, Logsdon CD. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J National Cancer Inst 2006;98:1806–1818.
Kostova N, Zlateva S, Ugrinova I, Pasheva E. The expression of HMGB1 protein and its receptor RAGE in human malignant tumors. Mol Cell Biochem 2010;337:251–258.
Tang D, Lotze MT, Zeh HJ, Kang R. The redox protein HMGB1 regulates cell death and survival in cancer treatment. Autophagy 2010;16:1181–1183.
Acknowledgments
This work was generously supported by the I.W. Foundation and an institutional Ruth L. Kirschstein National Research Service Award (T32 HL 007854–14).
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussant
Dr. Emina H. Huang (Gainesville, FL): Congratulations, Dr. DiNorcia, for continued excellent work under the guidance of Drs. Ann Marie Schmidt and John Allendorf. Your presentation today is the culmination of a tremendous labor of breeding, genotyping, and pathologic dissection and you are commended for your efforts.
In your discussion, you refer to the issues that mechanism in your studies is only inferred. With the recent advances in combining molecular pathways and signatures from malignancies such as melanoma and non-small cell lung cancer in efforts to provide targeted treatment, I have three questions:
1. Are either soluble RAGE or a RAGE small molecule inhibitor, or cromolyn or other S100P antagonists potential treatments for pancreatic cancer?
2. Have you tried these interventions early on in the Tuveson mouse model?
3. Do you see a role for RAGE antagonism in those kindred with a familial pancreatic cancer or for those lacking metastatic disease, to prevent either metastases or recurrence?
Again, brilliant work and congratulations!
Closing Discussant
Dr. Joseph DiNorcia: Thank you, Dr. Huang, for your gracious comments and questions. RAGE inhibitors may be potential treatments for pancreatic cancer. It also is possible to envision inhibitors of downstream signaling as potential treatments once these post-receptor pathways are better defined. Given the complex role RAGE plays in differentially regulating cell survival and cell death, we have considerable more work to do prior to clinical application.
Targeting the ligand–RAGE axis also may be a strategy to improve the effectiveness of chemotherapy. For example, it has been shown that HMGB1 is released following tumor cell death and interacts with RAGE on neighboring tumor cells to promote survival. By inhibiting this ligand–RAGE interaction, we might be able to enhance a chemotherapeutic agent's ability to kill tumor cells.
We have treated a very small number of Pdx1-Cre; Kras G12D/+; p16 Ink4a−/− mice with sRAGE. Although we noted no differences in the incidence of carcinoma, there was a significantly decreased stromal reaction in sRAGE-treated mice compared to controls, suggesting that stromal cells play roles in tumorigenesis via RAGE-dependent pathways.
Finally, perhaps the most practical clinical application of RAGE antagonism is in pancreatic cancer prevention. For example, RAGE inhibition in patients with IPMN may delay or arrest tumor progression to carcinoma. Or, in patients with localized pancreatic cancer, RAGE antagonism may prevent invasion and metastases by inhibiting tumor cell adhesion and motility. The ligand–RAGE axis is an attractive target for potential prophylaxis and treatment of pancreatic cancer and thus remains an exciting area for further study.
Rights and permissions
About this article
Cite this article
DiNorcia, J., Lee, M.K., Moroziewicz, D.N. et al. RAGE Gene Deletion Inhibits the Development and Progression of Ductal Neoplasia and Prolongs Survival in a Murine Model of Pancreatic Cancer. J Gastrointest Surg 16, 104–112 (2012). https://doi.org/10.1007/s11605-011-1754-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-011-1754-9