Abstract
Introduction
National Comprehensive Cancer Network (NCCN) guidelines recommend hepatic resection and lymphadenectomy (LND) for gallbladder adenocarcinoma (GBA). We sought to evaluate compliance with these recommendations and to assess trends in the management and survival of patients with GBA.
Methods
Using Surveillance, Epidemiology and End Results (SEER)-Medicare-linked data, we identified 2,955 patients with GBA who underwent cancer-directed surgery from 1991 to 2005. We assessed clinicopathologic data, trends in surgical management, and survival.
Results
From 1991 to 2005, preoperative evaluation included CT (62%), MRI (6%), and PET (2%). Only 383 (13%) patients underwent radical resection/hepatectomy with a temporal increase over the study period (1991–1995, 12%; 1996–1999, 10%; 2000–2002, 12.0%; 2003–2005, 16%; P < 0.001). For patients undergoing radical resection/hepatectomy, LND ≥ 3 nodes was performed in 96 (3%) patients. Among patients who had LND, 47% had nodal metastasis. The overall 1-, 3-, and 5-year survival was 56%, 30%, and 21%. On multivariate analysis, radical resection/hepatectomy (hazard ratio (HR) = 0.71) and LND ≥ 3 nodes (HR = 0.56) were independently associated with increased survival. There was no significant improvement in survival over time (P = 0.60).
Conclusions
Compliance with NCCN guidelines for GBA remains poor. Survival of patients with surgically managed GBA has not improved over time.




Similar content being viewed by others
References
Horner MJ RL, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK (eds). SEER Cancer Statistics Review, 1975–2006, National Cancer Institute. Bethesda, MD. Available at: http://seer.cancer.gov/csr/1975_2006/. Based on November 2008 SEER data submission, posted to the SEER web site, 2009.
Shih SP, Schulick RD, Cameron JL, et al. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg 2007; 245(6):893–901.
Fong Y, Malhotra S. Gallbladder cancer: recent advances and current guidelines for surgical therapy. Adv Surg 2001; 35:1–20.
Foster JM, Hoshi H, Gibbs JF, et al. Gallbladder cancer: Defining the indications for primary radical resection and radical re-resection. Ann Surg Oncol 2007; 14(2):833–840.
Chan SY, Poon RT, Lo CM, et al. Management of carcinoma of the gallbladder: a single-institution experience in 16 years. J Surg Oncol 2008; 97(2):156–164.
Balachandran P, Agarwal S, Krishnani N, et al. Predictors of long-term survival in patients with gallbladder cancer. J Gastrointest Surg 2006; 10(6):848–854.
Dixon E, Vollmer CM, Jr., Sahajpal A, et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American Center. Ann Surg 2005; 241(3):385–394.
Pawlik TM, Gleisner AL, Vigano L, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg 2007; 11(11):1478–86; discussion 1486–7.
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines™) Hepatobiliary Cancers. (Version 1.2010). © 2010. Available at: www.NCCN.org. Accessed 8 April 2010.
American Joint Commission on Cancer (2010) American Cancer Society, 7th ed. New York, NY: Springer
Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg 1994; 219(3):275–280.
Kokudo N, Makuuchi M, Natori T, et al. Strategies for surgical treatment of gallbladder carcinoma based on information available before resection. Arch Surg 2003; 138(7):741–750; discussion 750.
D’Angelica M, Brennan MF, Fortner JG, et al. Ninety-six five-year survivors after liver resection for metastatic colorectal cancer. J Am Coll Surg 1997; 185(6):554–559.
Konstantinidis IT, Deshpande V, Genevay M, et al. Trends in presentation and survival for gallbladder cancer during a period of more than 4 decades: a single-institution experience. Arch Surg 2009; 144(5):441–447; discussion 447.
Wise PE, Shi YY, Washington MK, et al. Radical resection improves survival for patients with pT2 gallbladder carcinoma. Am Surg 2001; 67(11):1041–1047.
Jensen EH, Abraham A, Habermann EB, et al. A critical analysis of the surgical management of early-stage gallbladder cancer in the United States. J Gastrointest Surg 2009; 13(4):722–727.
Jensen EH, Abraham A, Jarosek S, et al. Lymph node evaluation is associated with improved survival after surgery for early stage gallbladder cancer. Surgery 2009; 146(4):706–711; discussion 711–3.
Coburn NG, Cleary SP, Tan JC, et al. Surgery for gallbladder cancer: a population-based analysis. J Am Coll Surg 2008; 207(3):371–382.
Cooper GS, Virnig B, Klabunde CN, et al. Use of SEER-Medicare data for measuring cancer surgery. Med Care 2002; 40(8 Suppl):IV-43-8.
National Cancer Institute D, Surveillance Research Program, Cancer Statistics Branch. Surveillance, epidemiology, and end results (SEER) program. Available at: (http://www.seer.cancer.gov). SEER* Stat database: Incidence, SEER 17 regs public-use, Nov 2005 sub (1973–2003 varying), linked to county attributes, total US, 1969–2003 counties. Released April 2006, based on the November 2005 submission.
Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002; 40(8 Suppl):IV-3-18.
Fritz AG. International Classification of Diseases for Oncology: ICD-O, 3rd ed. Geneva: World Health Organization 2000.
American Joint Commission on Cancer, American Cancer Society. 6th ed. New York: Springer, 2002
SEER program coding and staging manual. Bethesda, MD: National Cancer Institute, 2007.
Morris AM, Baldwin LM, Matthews B, et al. Reoperation as a quality indicator in colorectal surgery: a population-based analysis. Ann Surg 2007; 245(1):73–79.
Potosky AL, Warren JL, Riedel ER, et al. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care 2002; 40(8 Suppl):IV-62-8.
Kaplan EL MP. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53:457–481.
Cox D. Regression models and life tables. J R Stat Soc B 1972; 34:187–220.
Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression modeling of time to event data. 2nd ed. New York: John Wiley and Sons, Inc., 1999.
May S, Hosmer DW. A simplified method of calculating an overall goodness-of-fit test for the Cox proportional hazards model. Lifetime Data Anal 1998; 4(2):109–120.
Agresti A. Introduction to categorical data analysis. New York: Wiley, 1996. pp. 231–236.
Nathan H, Pawlik TM. Limitations of claims and registry data in surgical oncology research. Ann Surg Oncol 2008; 15(2):415–423.
Kim SJ, Lee JM, Lee JY, et al. Accuracy of preoperative T-staging of gallbladder carcinoma using MDCT. AJR Am J Roentgenol 2008; 190(1):74–80.
Rao ND, Gulati MS, Paul SB, et al. Three-dimensional helical computed tomography cholangiography with minimum intensity projection in gallbladder carcinoma patients with obstructive jaundice: comparison with magnetic resonance cholangiography and percutaneous transhepatic cholangiography. J Gastroenterol Hepatol 2005; 20(2):304–308.
Park HS, Lee JM, Choi HK, et al. Preoperative evaluation of pancreatic cancer: comparison of gadolinium-enhanced dynamic MRI with MR cholangiopancreatography versus MDCT. J Magn Reson Imaging 2009; 30(3):586–595.
Manfredi R, Mehrabi S, Motton M, et al. MR imaging and MR cholangiopancreatography of multifocal intraductal papillary mucinous neoplasms of the side branches: MR pattern and its evolution. Radiol Med 2008; 113(3):414–428.
Guiu B, Loffroy R, Ben Salem D, et al. Combined SPIO-gadolinium magnetic resonance imaging in cirrhotic patients: negative predictive value and role in screening for hepatocellular carcinoma. Abdom Imaging 2008; 33(5):520–528.
Hueman MT, Vollmer CM, Jr., Pawlik TM. Evolving treatment strategies for gallbladder cancer. Ann Surg Oncol 2009; 16(8):2101–2115.
Corvera CU, Blumgart LH, Akhurst T, et al. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg 2008; 206(1):57–65.
Pawlik TM, Choti MA. Biology dictates prognosis following resection of gallbladder carcinoma: sometimes less is more. Ann Surg Oncol. 2009; 16(4):787–788.
D’Angelica M, Dalal KM, DeMatteo RP, et al. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol 2009; 16(4):806–816.
Tsao JI, Loftus JP, Nagorney DM, et al. Trends in morbidity and mortality of hepatic resection for malignancy. A matched comparative analysis. Ann Surg 1994; 220(2):199–205.
Thompson HH, Tompkins RK, Longmire WP, Jr. Major hepatic resection. A 25-year experience. Ann Surg 1983; 197(4):375–388.
Bentrem DJ, Dematteo RP, Blumgart LH. Surgical therapy for metastatic disease to the liver. Annu Rev Med 2005; 56:139–156.
Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999; 230(3):309–318; discussion 318–21.
Bozzetti F, Gennari L, Regalia E, et al. Morbidity and mortality after surgical resection of liver tumors. Analysis of 229 cases. Hepatogastroenterology 1992; 39(3):237–241.
Taylor M, Forster J, Langer B, et al. A study of prognostic factors for hepatic resection for colorectal metastases. Am J Surg 1997; 173(6):467–471.
Capussotti L, Polastri R. Operative risks of major hepatic resections. Hepatogastroenterology 1998; 45(19):184–190.
Asiyanbola B, Chang D, Gleisner AL, et al. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg 2008; 12(5):842–851.
Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg 2000; 232(4):557–569.
Wright BE, Lee CC, Iddings DM, et al. Management of T2 gallbladder cancer: are practice patterns consistent with national recommendations? Am J Surg 2007; 194(6):820–825; discussion 825–6.
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussion
Discussant
Dr. Gerard V. Aranha (Maywood, IL): Dr. Mayo and his co authors from the Department of Surgery at Johns Hopkins University have reviewed SEER-Medicare linked data and concluded that the National Comprehensive Cancer Network guidelines for the surgical treatment of gallbladder cancer are not being followed by a majority of institutions in this country.
Unfortunately, this is not just true for gallbladder cancer but it is true for many other malignancies. Being a surgical oncologist, I’m pretty aware of that and in my opinion, it requires urgent attention and intervention.
I have the following questions.
In your paper, you state that those patients who had extended hepatectomy had a 20% mortality compared to those who had a regular hemihepatectomy, which was 5%. Were the extended hepatectomies done at a local community hospital, low- or high-volume center, and whom in gallbladder cancer do you think should have an extended hepatectomy?
How do we change the culture so that the NCCN guidelines will be followed in this country? Do you think that, at least in gallbladder cancer, that the patients who need surgical treatment should be referred to high-volume centers where maybe the guidelines are followed? Or do you believe that this should be done through the Commission on Cancer of the American College of Surgeons? I think this is a topic that you should address in your manuscript and needs attention.
I think you did a great job with your presentation, Dr. Mayo, and I commend it to the membership at large.
Closing Discussant
Dr. Skye C. Mayo: Thank you for your excellent questions and comments, Dr. Aranha. In our manuscript, we show that there was no difference in mortality between patients undergoing an extended hepatectomy versus a partial or hemihepatectomy. The number of patients who had a hepatectomy who suffered a peri-operative mortality was low. For instance, of the people that had an extended hepatectomy, one patient died post-operatively. Amongst the patients who had a partial hepatectomy or a hemihepatectomy, there were two peri-operative deaths. The numbers are very small and statistically fragile. I would be hesitant to draw conclusions from these data.
As for patients who should undergo a more extended procedure for clearance of their cancer, I believe the recent literature supports that an extended hepatectomy should not be performed on a routine basis for patients with gallbladder cancer, but instead should be performed only for certain patients to achieve clearance of their disease. An extended hepatectomy is associated with a higher morbidity, but it has not been shown to improve long-term survival. The recent literature has shown that it’s not the extent of resection that’s important, but rather the status of the surgical margin that influences patient outcome. I think the surgeon’s aim should be to achieve a microscopically complete resection with disease clearance and limit the resection at that.
As for the cultural change and the NCCN guidelines, I agree with you that it’s a very pressing issue and one that is very difficult to address. I think it has to start with standardization of documentation at a more national level and then be disseminated to the smaller hospitals and community centers. Checklists and guidelines should be developed that allow the surgeon and the hospital to collect these data prospectively to ensure that they are following the recommended guidelines appropriately.
In regards to patient referral to high-volume centers, I really wish that we could have looked at volume within the study, but within the SEER database there’s really no reliable measure of volume.
Discussant
Dr. Sharon Weber (Madison, WI): I am trying to put this in the context of what we see clinically. These are clearly unbelievably low numbers of patients that are getting referred on for definitive resection. But so often clinically what we see is the patient who had an incidental finding of gallbladder cancer after laparoscopic cholecystectomy, and we see them and obtain a re-staging MRI or CT scan and they are found to have metastatic disease, and therefore we never operate on them.
How would this dataset code that patient? Are those patients counted as having metastatic disease based on the imaging studies? Or is the pathologic staging based solely on the operation which they had, in that case, just a lap chole?
The second question I have for you is about predictors of patients that underwent radical resection. Did you do a multivariate logistical regression trying to sort this out a little more, to understand which patients actually were more likely to undergo radical resection for their gallbladder cancer?
Closing Discussant
Dr. Skye C. Mayo: Thank you for your questions, Dr. Weber. As for patients with metastatic disease being included in this database, one of our exclusion criteria was to use the SEER summary stages and the historical cancer stage to exclude patients with metastatic disease. Whether those patients are undergoing a laparoscopic cholecystectomy and later having metastatic disease added onto their record, I don’t believe that is the case. The SEER database collects tumor specific information at the time of diagnosis and treatment and records only if the patient had metastatic disease at the time of their diagnosis. I’m not certain how the database handles a patient that develops metastatic disease in the time period between their cholecystectomy and their referral for re-resection.
In regards to factors associated with patients who underwent a radical resection, we found that patients who were in the younger age quartile of our cohort, and patients who had a more recent operation were more likely to have had a radical resection.
Discussant
Dr. Henry Pitt (Indianapolis, IN): This analysis and presentation was very good. Your data suggest that an extended operation is warranted with T2 and T3 tumors. However, I didn’t see you comment on an extended operation for T1b tumors. I presume the data were not robust enough to answer this question. For your information, I just reviewed a meta-analysis of the literature that suggests that extended cholecystectomy is also warranted in T1b. Can you comment further from your analysis?
Closing Discussant
Dr. Skye C. Mayo: Thank you, Dr. Pitt for your question. In the data from recently published literature, in patients with T1b cancers, approximately 10% have residual disease in their gallbladder fossa that is found at their re-resection. Due to this percentage, many surgeons advocate radical cholecystectomy for those patients with a T1b cancer discovered after their initial cholecystectomy. In our data, not surprisingly, there was a survival benefit for patients who had a T1b cancer, but we chose to focus T2 and T3 for our discussion today. It’s more fully delineated in our paper.
Discussant
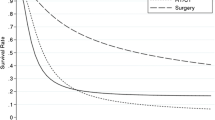
Dr. Fabrizio Michelassi (New York, NY): Nice presentation. My question relates to the survival curves according to extent of surgery in lymph node negative patients. I believe that you showed a graph with two distinct survival curves, one for patients who had undergone a radical resection, and the second one, for patients without a radical resection. Am I interpreting the data correctly?
Closing Discussant
Dr. Skye C. Mayo: Thank you, Dr. Michelassi. There were two different survival curves. The first one compared the survival impact of radical resection for patients with T2 versus T3 cancers. The second assessed the survival impact of lymphadenectomy on patients with T2 versus T3 cancers. It was irrespective of radical resection. We show an increase of 18 months median survival in patients with a T2 cancer who underwent a lymphadenectomy as indicated by their SEER-Medicare data.
Rights and permissions
About this article
Cite this article
Mayo, S.C., Shore, A.D., Nathan, H. et al. National Trends in the Management and Survival of Surgically Managed Gallbladder Adenocarcinoma Over 15 years: A Population-Based Analysis. J Gastrointest Surg 14, 1578–1591 (2010). https://doi.org/10.1007/s11605-010-1335-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-010-1335-3