Abstract
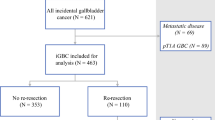
Re-resection for gallbladder carcinoma incidentally discovered after cholecystectomy is routinely advocated. However, the incidence of finding additional disease at the time of re-resection remains poorly defined. Between 1984 and 2006, 115 patients underwent re-resection at six major hepatobiliary centers for gallbladder carcinoma incidentally discovered during cholecystectomy. Data on clinicopathologic factors, operative details, TNM tumor stage, and outcome were collected and analyzed. Data on the incidence and location of residual/additional carcinoma discovered at the time of re-resection were also recorded. On pathologic analysis, T stage was T1 7.8%, T2 67.0%, and T3 25.2%. The median time from cholecystectomy to re-resection was 52 days. At the time of re-resection, hepatic surgery most often consisted of formal segmentectomy (64.9%). Patients underwent lymphadenectomy (LND) (50.5%) or LND + common bile duct resection (43.3%). The median number of lymph nodes harvested was 3 and did not differ between LND alone (n = 3) vs LND + common duct resection (n = 3) (P = 0.35). Pathology from the re-resection specimen noted residual/additional disease in 46.4% of patients. Of those patients staged as T1, T2, or T3, 0, 10.4, and 36.4%, respectively, had residual disease within the liver (P = 0.01). T stage was also associated with the risk of metastasis to locoregional lymph nodes (lymph node metastasis: T1 12.5%; T2 31.3%, T3 45.5%; P = 0.04). Cystic duct margin status predicted residual disease in the common bile duct (negative cystic duct, 4.3% vs positive cystic duct, 42.1%) (P = 0.01). Aggressive re-resection for incidental gallbladder carcinoma is warranted as the majority of patients have residual disease. Although common duct resection does not yield a greater lymph node count, it should be performed at the time of re-resection for patients with positive cystic duct margins because over one-third will have residual disease in the common bile duct.






Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin 2006;56:106–130.
Shirai Y, Yoshida K, Tsukada K, Muto T. Inapparent carcinoma of the gallbladder. An appraisal of a radical second operation after simple cholecystectomy. Ann Surg 1992;215:326–331.
Thorbjarnarson B, Glenn F. Carcinoma of the gallbladder. Cancer 1959;12:1009–1015.
Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg 1994;219:275–280.
Piehler JM, Crichlow RW. Primary carcinoma of the gallbladder. Surg Gynecol Obstet 1978;147:929–942.
Tsukada K, Hatakeyama K, Kurosaki I, Uchida K, Shirai Y, Muto T, et al. Outcome of radical surgery for carcinoma of the gallbladder according to the TNM stage. Surgery 1996;120:816–821.
Bartlett DL, Fong Y, Fortner JG, Brennan MF, Blumgart LH. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg 1996;224:639–646.
Fong Y, Heffernan N, Blumgart LH. Gallbladder carcinoma discovered during laparoscopic cholecystectomy: aggressive reresection is beneficial. Cancer 1998;83:423–427.
Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the gallbladder. Histologic types, stage of disease, grade, and survival rates. Cancer 1992;70:1493–1497.
Chijiiwa K, Tanaka M. Carcinoma of the gallbladder: an appraisal of surgical resection. Surgery 1994;115:751–756.
Muratore A, Polastri R, Bouzari H, Vergara V, Capussotti L. Radical surgery for gallbladder cancer: a worthwhile operation? Eur J Surg Oncol 2000;26:160–163.
Foster JM, Hoshi H, Gibbs JF, Iyer R, Javle M, Chu Q, et al. Gallbladder cancer: defining the indications for primary radical resection and radical re-resection. Ann Surg Oncol 2007;14:833–840.
Benoist S, Panis Y, Fagniez PL. Long-term results after curative resection for carcinoma of the gallbladder. French University Association for Surgical Research. Am J Surg 1998;175:118–122.
Donohue JH, Nagorney DM, Grant CS, Tsushima K, Ilstrup DM, Adson MA. Carcinoma of the gallbladder. Does radical resection improve outcome? Arch Surg 1990;125:237–241.
Yamaguchi K, Tsuneyoshi M. Subclinical gallbladder carcinoma. Am J Surg 1992;163:382–386.
Gallbladder. In Greene FL, Page DL, Fleming ID, et al., eds. American Joint Committee on Cancer Staging manual. New York, NY: Springer, 2002. pp 139–142.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Am Stat Assoc J 1958;53:457–480.
Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg 2000;232:557–569.
Wakai T, Shirai Y, Yokoyama N, Ajioka Y, Watanabe H, Hatakeyama K. Depth of subserosal invasion predicts long-term survival after resection in patients with T2 gallbladder carcinoma. Ann Surg Oncol 2003;10:447–454.
Yasui K, Shimizu Y. Surgical treatment for metastatic malignancies. Anatomical resection of liver metastasis: indications and outcomes. Int J Clin Oncol 2005;10:86–96.
Yamamoto J, Saiura A, Koga R, Seki M, Ueno M, Oya M, et al. Surgical treatment for metastatic malignancies. Nonanatomical resection of liver metastasis: indications and outcomes. Int J Clin Oncol 2005;10:97–102.
Zorzi D, Mullen JT, Abdalla EK, Pawlik TM, Andres A, Muratore A, et al. Comparison between hepatic wedge resection and anatomic resection for colorectal liver metastases. J Gastrointest Surg 2006;10:86–94.
Chijiiwa K, Nakano K, Ueda J, Noshiro H, Nagai E, Yamaguchi K, et al. Surgical treatment of patients with T2 gallbladder carcinoma invading the subserosal layer. J Am Coll Surg 2001;192:600–607.
Shimada H, Endo I, Togo S, Nakano A, Izumi T, Nakagawara G. The role of lymph node dissection in the treatment of gallbladder carcinoma. Cancer 1997;79:892–899.
Nakamura S, Sakaguchi S, Suzuki S, Muro H. Aggressive surgery for carcinoma of the gallbladder. Surgery 1989;106:467–473.
Shimizu Y, Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, et al. Should the extrahepatic bile duct be resected for locally advanced gallbladder cancer? Surgery 2004;136:1012–1017; discussion 1018.
Kosuge T, Sano K, Shimada K, Yamamoto J, Yamasaki S, Makuuchi M. Should the bile duct be preserved or removed in radical surgery for gallbladder cancer? Hepatogastroenterology 1999;46:2133–2137.
Chijiiwa K, Tanaka M. Indications for and limitations of extended cholecystectomy in the treatment of carcinoma of the gall bladder. Eur J Surg 1996;162:211–216.
Wanebo HJ, Castle WN, Fechner RE. Is carcinoma of the gallbladder a curable lesion? Ann Surg 1982;195:624–631.
Ouchi K, Owada Y, Matsuno S, Sato T. Prognostic factors in the surgical treatment of gallbladder carcinoma. Surgery 1987;101:731–737.
Chijiiwa K, Noshiro H, Nakano K, Okido M, Sugitani A, Yamaguchi K, et al. Role of surgery for gallbladder carcinoma with special reference to lymph node metastasis and stage using western and Japanese classification systems. World J Surg 2000;24:1271–1276; discussion 1277.
Acknowledgement
Ana Gleisner is supported by a UICC American Cancer Society Beginning Investigators Fellowship funded by the American Cancer Society and by a grant from CAPES (Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior).
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussion
Bryan M. Clary, M.D. (Durham, NC): That was a very nice presentation. This paper is important, I think, for a couple of reasons. Number one, I think it does help to cast a pall over the concept that you need to do a bile duct excision just to get a better lymph node clearance in patients who do not clearly have cystic duct involvement. The second important concept is that it does help, I think, to better define those patients who have residual disease and the likely sites of their residual disease. With its design, it does not help, in my mind, to define whether these interventions are necessarily the reason why these patients demonstrate long-term survival. So the value of the intervention is a little less clear, again, given the retrospective nature of this design. I have some questions for Tim.
Number one, you made conclusions about the extent of the hepatic resection. Now, unless you are very clearly taking hepatic segment 4B and 5, in general, a segment 4B/5 resection as most people practice, is just a big glorified wedge excision. Can you comment on whether this was truly a formal segmental resection?
You did not really comment on adjuvant therapy, and I hope that you would, especially in light of your 5-year survival in your node positive patients, which is better than most nihilistic individuals tend to expect.
In reading through the manuscript, you had 28 patients who had peritoneal and liver metastasis, yet, you only had 18 patients who did not undergo a radical re-excision during that exploration. Were you resecting metastatic disease in the other ten patients?
Lastly you do have a population of patients who underwent simple cholecystectomy alone, actually 17 of those 33 patients. Did you actually look at their survival compared to your radical re-excision staged patients?
Thanks again Tim for a great presentation.
Timothy M. Pawlik, M.D. (Baltimore, MD): Thank you Bryan for your questions. I would like to address your last question first. The objective of this study was not to look at long-term survival and the survival “value” of the actual second operation. Whether cholecystectomy alone vs radical re-resection yields improved overall survival is difficult to address. As we stated in the paper, the second operation not only has a therapeutic effect, it also has a staging effect. So, it is hard to know if someone who had a simple cholecystectomy and was T2 Nx is truly similar to someone who had a radical re-resection and is T2 with known lymph node status. In other words, are these two patient cohorts truly the same stage or is one better staged due to the difference in lymph node evaluation? As you can see from our work, in T2 patients, a fair number of T2 patients will have lymph node metastases. So, when comparing T2 patients after simple cholecystectomy vs those who underwent re-resection, it is hard to know if you are comparing apple to apples or apples to oranges. As such, we wanted to avoid the question of whether the second operation provided a direct survival benefit. Rather, we tried to indirectly address the relative benefit of the re-resection by the notion of how much residual disease is being left behind by performing only a simple cholecystectomy.
I have to say it was my bias that these re-resections for patients with gallbladder cancer were not going to yield much residual disease. However, as we showed, over 40% of patients did indeed have additional disease discovered at the time of the second operation. These data again suggest that a staging migration phenomena can occur based on the findings of the second operation. They also suggest that re-resection of patients with T2 or T3 disease should continue to be recommended.
As far as the extent of hepatic resection, I completely agree with you. This was self-reported, so I cannot tell you for sure that these segmentectomies of 4B and 5 were truly anatomic resections. As you know, there are data in the literature on colorectal hepatic metastasis and hepatocellular carcinoma regarding the topic of anatomic vs nonanatomic resection. We have previously published on hepatic resection of colorectal metastasis and reported that an anatomic resection was not necessary. As long as the margins were negative, the results were the same. Similarly, in our current study on hepatic resection for gallbladder cancer, an anatomic vs nonanatomic resection did not affect outcome. However, what did matter was whether the surgeon was able to obtain an R0 resection. Whether the R0 resection could be accomplished by an anatomic vs nonanatomic resection did not seem to matter.
Unfortunately I cannot comment on adjuvant therapy. We were not able to collect these data because, as you can imagine, it was difficult to obtain adjuvant data from six centers that spanned the United States, Europe, and South America.
Finally, you are correct. There were ten patients who underwent a re-resection who had metastatic disease. Some of the centers in Brazil and in Italy did resect a couple of patients who had limited metastatic disease.
Charles M. Vollmer, Jr., M.D. (Boston, MA): Great talk again. I have got two questions about this. The first is that group of 33 patients which you did not seem to focus here, and if I heard Bryan right, he said that there were 17 that got a simple cholecystectomy alone; what happened to the other group in that 33? What kind of operations were they getting? Is this a scenario where they were getting a resection of the gallbladder, there is an obvious tumor found, and then someone decides to convert to a bigger operation? I would be curious to know what kind of results in survival came from that operation.
The second thing is in your cohort that did get the re-resection; do you have any data on the interval period between the original cholecystectomy and then the operation? Because some feel that you can wait these things out and restage many months later to get a better staging effect, others will go directly to the operation within a week or two of the finding. So do you know anything about that?
Thanks.
Dr. Pawlik: Charles, thanks so much for your comments. The median time between cholecystectomy and radical re-resection was 52 days. The range was fairly wide, but the median was 52 days.
Your other question was about those 33 patients who were treated “definitively” at the time of the initial operation. These patients underwent a myriad of operations. Some had a simple cholecystectomy, while others had a more extensive resection. It was largely based on T stage, with some T2 and the T3 patients actually undergoing hepatic resection. I believe 3 of the 33 patients had a common bile duct resection and lymphadenectomy. So, indeed, there was really a mix of the type of operations performed—even when the surgeon chose to definitively treat the gallbladder cancer at the time of the initial cholecystectomy.
What we did not do, and perhaps we should have, is investigate how patients who had the “definitive” surgical procedure done at the time of the initial cholecystectomy compared with those patients who had a staged operation.
Rights and permissions
About this article
Cite this article
Pawlik, T.M., Gleisner, A.L., Vigano, L. et al. Incidence of Finding Residual Disease for Incidental Gallbladder Carcinoma: Implications for Re-resection. J Gastrointest Surg 11, 1478–1487 (2007). https://doi.org/10.1007/s11605-007-0309-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-007-0309-6