Abstract
Introduction
Chemokine receptors may regulate the progression and metastasis of invasive malignancies. There are little data, however, regarding their role in premalignant lesions. Our objective was to determine the role of CC chemokine receptor 9 (CCR9) in pancreatic intraepithelial neoplasia (PanIN).
Methods
Human and murine formalin-fixed paraffin-embedded (FFPE) PanIN specimens were assessed for CCR9 expression. The established murine PanIN, invasive pancreatic cancer (5143PDA) and liver metastasis (5143LM) cell lines, and human pancreatic cancer cell line (PANC-1) were obtained to verify CCR9 expression and function.
Results
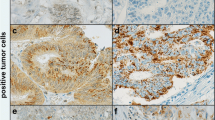
Immunohistochemistry of FFPE specimens demonstrated CCR9 expression in both murine and human PanIN lesions. CCR9 expression in murine and human cell lines was verified by Western blot assay, immunofluorescence, and flow cytometry. CCR9 function was demonstrated by in vitro exposure to CCL25, the selective CCR9 ligand, which resulted in significantly increased cell proliferation in PanIN and pancreatic cancer cell lines.
Conclusions
This is the first report of chemokine receptor CCR9 expression in murine and human PanIN tissues. Our results demonstrate enhanced PanIN and pancreatic cancer cell proliferation with activation of CCR9 by its selective ligand CCL25. CCR9 may prove to be a novel therapeutic target for PanIN and its progression to invasive cancer.





Similar content being viewed by others
References
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W. Erlotinib plus gemcitabine compared to gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960–1966.
Phillip P, Benedetti J, Fenoglio-Preiser C, Zalupski M, Lenz H, O’Reilly E, Wong R, Atkins J, Abruzzese J, Blanke C. Phase III study of gemcitabine plus cetuximab versus gemcitabine in patients with locally advanced or metastatic pancreatic adenocarcinoma SWOG S0205 study. J Clin Oncol 2007;25(18S):4509.
Kindler H, Niedzwiecki D, Hollis D, Oraefo E, Schrag D, Hurwitz H, McLeod HL, Mulcahy MF, Schilsky RL, Goldberg RM. A double-blind, placebo-controlled, randomized phase III trial of gemcitabine plus bevacizumab versus gemcitabine plus placebo in patients with advanced pancreatic cancer: a preliminary analysis of Cancer and Leukemia Group B (CALGB) 80303. J Clin Oncol 2007;25(18S):4508.
Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J, Moore MJ. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 2009;27:2231–2237.
Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Klöppel G, Longnecker DS, Lüttges J, Offerhaus GJ. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol 2001;25:579–586.
Klein WM, Hruban RH, Klein-Szanto AJ, Wilentz RE. Direct correlation between proliferative activity and dysplasia in pancreatic intraepithelial neoplasia (PanIN): additional evidence for a recently proposed model of progression. Mod Pathol 2002;15:441–447.
Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CVE, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003;4:437–450.
Thomas RM, Kim J, Revelo-Penafiel MP, Angel R, Dawson DW, Lowy AM. The chemokine receptor CXCR4 is expressed in pancreatic intraepithelial neoplasia. Gut 2008;57:1555–1560.
Vicari AP, Figueroa DJ, Hedrick JA, Foster JS, Singh KP, Menon S, Copeland NG, Gilbert DJ, Jenkins NA, Bacon KB, Zlotnik A. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity 1997;7:291–301.
Zaballos A, Gutiérrez J, Varona R, Ardavín C, Márquez G. Cutting edge: identification of the orphan chemokine receptor GPR-9–6 as CCR9, the receptor for the chemokine TECK. J Immunol 1999;162:5671–5675.
Wurbel MA, Malissen M, Guy-Grand D, Meffre E, Nussenzweig MC, Richelme M, Carrier A, Malissen B. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor gamma/delta (+) gut intraepithelial lymphocytes. Blood 2001;98:2626–2632.
Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB, Genovese MC, Wardlaw AJ, Greenberg HB, Parker CM, Butcher EC, Andrew DP, Agace WW. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med 2000;192:761–768.
Zabel BA, Agace WW, Campbell JJ, Heath HM, Parent D, Roberts AI, Ebert EC, Kassam N, Qin S, Zovko M, LaRosa GJ, Yang LL, Soler D, Butcher EC, Ponath PD, Parker CM, Andrew DP. Human G protein-coupled receptor GPR-9–6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J Exp Med 1999;190:1241–1256.
Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med 2003;198:963–969.
Letsch A, Keilholz U, Schadendorf D, Assfalg G, Asemissen AM, Thiel E, Scheibenbogen C et al. Functional CCR9 expression is associated with small intestinal metastasis. J Invest Dermatol 2004;122:685–690.
Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005;7:469–483.
Liu N, Furukawa T, Kobari M, Tsao MS. Comparative phenotypic studies of duct epithelial cell lines derived from normal human pancreas and pancreatic carcinoma. Am J Pathol 1998;153:263–269.
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659–1672.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–2342.
Balkwill F. Cancer and the chemokine network. Nat Rev Cancer 2004;4:540–550.
Ben-Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp Metastasis 2008;25:345–356.
Honczarenko M, Douglas RS, Mathias C, Lee B, Ratajczak MZ, Silberstein LE. SDF-1 responsiveness does not correlate with CXCR4 expression levels of developing human bone marrow cells. Blood 1999;94:2990–2998.
Mitra P, De A, Ethier MF, Mimori K, Kodys K, Shibuta K, Mori M, Madison JM, Miller-Graziano C, Barnard GF. Loss of chemokine SDF-1alpha-mediated CXCR4 signalling and receptor internalization in human hepatoma cell line HepG2. Cell Signal 2001;13:311–319.
Marchesi F, Monti P, Leone BE, Zerbi A, Vecchi A, Piemonti L, Mantovani A, Allavena P. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res 2004;64:8420–8427.
Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001;410:50–56.
Acknowledgements
Presented at the 50th Annual Meeting of The Society for Surgery of the Alimentary Tract; Chicago IL, 2009.
Financial Support
Susan E. Riley Foundation and STOP Cancer Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussion
Dr. Brian A. Mailey, Presenter (City of Hope, Duarte, CA, USA)
Discussant
Dr. Syed Ahmad (Cincinnati, OH, USA): Your group has previously presented on CXCR4 in PanIN cells and demonstrated advancing degree of expression as PanIN progresses from 1 to 3 and that activation of CXCR4 increased growth and proliferation. And in this current study, you’ve demonstrated that CCR9 is present in PanIN cells. With that background, I have a few questions.
Were you able to demonstrate a similar finding in terms of differential degree of expression from PanIN‑1 to PanIN‑3? Was there a difference in expression in primary pancreatic cancers versus liver metastases? Have you looked at that or is that something you are planning to do?
The second question I have is, how do you explain the presence of CCR9 on normal ductal epithelial cells? In fact in the western that you demonstrated, it appeared that the expression of CCR9 was higher in normal cells than in PanIN cells. Have you done any assays to see if ductal cells proliferate when exposed to the ligand for CCR9?
Finally, the last question I have is, if you are proposing this as a mechanism of progression, how do you explain that mechanism? Where does the ligand come from? And do you see this as more of a chemo preventive target or a chemotherapeutic target?
Closing Discussant
Dr. Brian Mailey: We are very interested in the CCR9 receptor, but we are still in the early stages of investigation. The question that you raised about the increase in progression of expression in CXCR4 and what we have seen in CCR9, is that it is not exactly the same. We don’t see a progression in CCR9 expression from PanIN‑1 to PanIN‑3, although there are pending studies which may help further define this.
The second question about the HPDE cell line; we did demonstrate that CCR9 is present in normal pancreatic ductal cells. The function of CCR9 in these cells? We can speculate on what it may potentially do, although we don’t have a firm answer for that yet. I think that the western blot demonstrates that CCR9 expression is at least as high in PanIN as it is in HPDE. It will be interesting to determine if CCR9 does play a function in normal cells, and if the downstream signaling functions differently in pathologic cells.
(Questioner from the floor not using a microphone.)
The ligand is one of the most interesting aspects of this receptor, which really stimulated our interest in investigating it further when we discovered CCR9 on the microarray analysis. It is produced by the small bowel as I mentioned, and this was part of our hypothesis, that the ligand may be released in a paracrine manner to stimulate pancreatic cancer cells.
(Questioner from the floor not using a microphone.)
It may be a possible explanation for the extremely poor survival in pancreatic cancer, even for patients with early stage lesions. I think that chemo‑preventive versus therapeutic measures are still forthcoming. I think it’s a little bit early for us to tell.
Rights and permissions
About this article
Cite this article
Shen, X., Mailey, B., Ellenhorn, J.D.I. et al. CC Chemokine Receptor 9 Enhances Proliferation in Pancreatic Intraepithelial Neoplasia and Pancreatic Cancer Cells. J Gastrointest Surg 13, 1955–1962 (2009). https://doi.org/10.1007/s11605-009-1002-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-009-1002-8