Abstract
Introduction
Organ complications like biliary or duodenal stenosis as well as intractable pain are current indications for surgery in patients with chronic pancreatitis (CP). We present here our experience with pancreatic resection for CP and focus on the long-term outcome after surgery regarding pain, exocrine/endocrine pancreatic function, and the control of organ complications in 224 patients with a median postoperative follow-up period of 56 months.
Methods
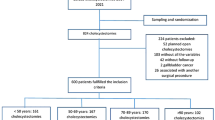
During 11 years 272 pancreatic resections were performed in our institution for CP. Perioperative mortality was 1%. Follow-up data using at least standardized questionnaires were available in 224 patients. The types of resection in these 224 patients were Whipple (9%), pylorus-preserving pancreato-duodenectomy (PD) (PPPD; 40%), duodenum-preserving pancreatic head resection (DPPHR; 41%, 50 Frey, 42 Beger), distal (9%) and two central pancreatic resections. Eighty-six of the patients were part of a randomized study comparing PPPD and DPPHR. The perioperative and follow-up (f/up) data were prospectively documented. Exocrine insufficiency was regarded as the presence of steatorrhea and/or the need for oral enzyme supplementation. Multivariate analysis was performed using binary logistic regression.
Results
Perioperative surgical morbidity was 28% and did not differ between the types of resection. At last f/up 87% of the patients were pain-free (60%) or had pain less frequently than once per week (27%). Thirteen percent had frequent pain, at least once per week (no difference between the operative procedures). A concomitant exocrine insufficiency and former postoperative surgical complications were the strongest independent risk factors for pain and frequent pain at follow-up. At the last f/up 65% had exocrine insufficiency, half of them developed it during the postoperative course. The presence of regional or generalized portal hypertension, a low preoperative body mass index, and a longer preoperative duration of CP were independent risk factors for exocrine insufficiency. Thirty-seven percent of the patients without preoperative diabetes developed de novo diabetes during f/up (no risk factor identified). Both, exocrine and endocrine insufficiencies were independent of the type of surgery. Median weight gain was 2 kg and higher in patients with preoperative malnutrition and in patients without abdominal pain. After PPPD, 8% of the patients had peptic jejunal ulcers, whereas 4% presented with biliary complications after DPPHR. Late mortality was analyzed in 233 patients. Survival rates after pancreatic resection for CP were 86% after 5 years and 65% after 10 years.
Conclusions
Pancreatic resection leads to adequate pain control in the majority of patients with CP. Long-term outcome does not depend on the type of surgical procedure but is in part influenced by severe preoperative CP and by postoperative surgical complications (regarding pain). A few patients develop procedure-related late complications. Late mortality is high, probably because of the high comorbidity (alcohol, smoking) in many of these patients.
Similar content being viewed by others
References
Rosch T, Daniel S, Scholz M, Huibregtse K, Smits M, Schneider T, Ell C, Haber G, Riemann JF, Jakobs R, Hintze R, Adler A, Neuhaus H, Zavoral M, Zavada F, Schusdziarra V, Soehendra N. Endoscopic treatment of chronic pancreatitis: a multicenter study of 1000 patients with long-term follow-up. Endoscopy 2002;34:765–771.
Delhaye M, Arvanitakis M, Verset G, Cremer M, Deviere J. Long-term clinical outcome after endoscopic pancreatic ductal drainage for patients with painful chronic pancreatitis. Clin Gastroenterol Hepatol 2004;2:1096–1106.
Steer ML, Waxman I, Freedman S. Chronic pancreatitis. N Engl J Med 1995;332:1482–1490.
Di Sebastiano P, di Mola FF, Bockman DE, Friess H, Buchler MW. Chronic pancreatitis: the perspective of pain generation by neuroimmune interaction. Gut 2003;52:907–911.
Beger HG, Buchler M. Duodenum-preserving resection of the head of the pancreas in chronic pancreatitis with inflammatory mass in the head. World J Surg 1990;14:83–87.
Markowitz JS, Rattner DW, Warshaw AL. Failure of symptomatic relief after pancreaticojejunal decompression for chronic pancreatitis. Strategies for salvage. Arch Surg 1994;129:374–379.
Beger HG, Krautzberger W, Bittner R, Buchler M, Limmer J. Duodenum-preserving resection of the head of the pancreas in patients with severe chronic pancreatitis. Surgery 1985;97:467–473.
Frey CF, Smith GJ. Description and rationale of a new operation for chronic pancreatitis. Pancreas 1987;2:701–707.
Traverso LW, Longmire WP, Jr. Preservation of the pylorus in pancreaticoduodenectomy. Surg Gynecol Obstet 1978;146:959–962.
Sakorafas GH, Farnell MB, Nagorney DM, Sarr MG, Rowland CM. Pancreatoduodenectomy for chronic pancreatitis: long-term results in 105 patients. Arch Surg 2000;135:517–523.
Schafer M, Mullhaupt B, Clavien PA. Evidence-based pancreatic head resection for pancreatic cancer and chronic pancreatitis. Ann Surg 2002;236:137–148.
Strate T, Taherpour Z, Bloechle C, Mann O, Bruhn JP, Schneider C, Kuechler T, Yekebas E, Izbicki JR. Long-term follow-up of a randomized trial comparing the beger and frey procedures for patients suffering from chronic pancreatitis. Ann Surg 2005;241:591–598.
Traverso LW, Kozarek RA. Pancreatoduodenectomy for chronic pancreatitis: anatomic selection criteria and subsequent long-term outcome analysis. Ann Surg 1997;226:429–435.
Buchler MW, Friess H, Muller MW, Wheatley AM, Beger HG. Randomized trial of duodenum-preserving pancreatic head resection versus pylorus-preserving Whipple in chronic pancreatitis. Am J Surg 1995;169:65–69.
Adam U, Makowiec F, Riediger H, Schareck WD, Benz S, Hopt UT. Risk factors for complications after pancreatic head resection. Am J Surg 2004;187:201–208.
Nealon WH, Matin S. Analysis of surgical success in preventing recurrent acute exacerbations in chronic pancreatitis. Ann Surg 2001;233:793–800.
Lucas CE, McIntosh B, Paley D, Ledgerwood AM, Vlahos A. Surgical decompression of ductal obstruction in patients with chronic pancreatitis. Surgery 1999;126:790–795.
Sohn TA, Campbell KA, Pitt HA, Sauter PK, Coleman JA, Lillemo KD, Yeo CJ, Cameron JL. Quality of life and long-term survival after surgery for chronic pancreatitis. J Gastrointest Surg 2000;4:355–364.
Jimenez RE, Fernandez-Del Castillo C, Rattner DW, Chang Y, Warshaw AL. Outcome of pancreaticoduodenectomy with pylorus preservation or with antrectomy in the treatment of chronic pancreatitis. Ann Surg 2000;231:293–300.
Beger HG, Schlosser W, Friess HM, Buchler MW. Duodenum-preserving head resection in chronic pancreatitis changes the natural course of the disease: a single-center 26-year experience. Ann Surg 1999;230:512–519.
Ammann RW, Muellhaupt B. The natural history of pain in alcoholic chronic pancreatitis. Gastroenterology 1999;116:1132–1140.
Layer P, Yamamoto H, Kalthoff L, Clain JE, Bakken LJ, DiMagno EP. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology 1994;107:1481–1487.
Malka D, Hammel P, Sauvanet A, Rufat P, O’Toole D, Bardet P, Belghiti J, Bernades P, Ruszniewski P, Levy P. Risk factors for diabetes mellitus in chronic pancreatitis. Gastroenterology 2000;119:1324–1332.
Sakorafas GH, Farnell MB, Farley DR, Rowland CM, Sarr MG. Long-term results after surgery for chronic pancreatitis. Int J Pancreatol 2000;27:131–142.
Sakorafas GH, Sarr MG. Pancreatic cancer after surgery for chronic pancreatitis. Dig Liver Dis 2003;35:482–485.
Witzigmann H, Max D, Uhlmann D, Geissler F, Schwarz R, Ludwig S, Lohmann T, Caca K, Keim V, Tannapfel A, Hauss J. Outcome after duodenum-preserving pancreatic head resection is improved compared with classic Whipple procedure in the treatment of chronic pancreatitis. Surgery 2003;134:53–62.
Evans JD, Wilson PG, Carver C, Bramhall SR, Buckels JA, Mayer AD, McMaster P, Neoptolemos JP. Outcome of surgery for chronic pancreatitis. Br J Surg 1997;84:624–629.
Greenlee HB, Prinz RA, Aranha GV. Long-term results of side-to-side pancreaticojejunostomy. World J Surg 2006;14:70–76.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCUSSION
Dr. W. Nealon (Galveston, TX): Dr. Kaufman, Dr. Joseph, members and guests. The authors from Freiburg have contributed yet another thorough review of their outcomes in the management of chronic pancreatitis. They report today their experience with 272 resections, including pancreaticoduodenectomy, classic Whipple, and duodenum-preserving pancreatic head resection either by the Frey or Beger technique, and they provide detailed follow-up data, including measures of pain relief, functional derangements, and nutritional outcomes.
Their operative mortality of 1% is striking, particularly considering their inclusion of patients with portal vein or splenic vein thrombosis, which they have seen in 25% of their resected patients, and often this entity raises considerably the risk for hemorrhage in these resections. Complete abolition of pain was achieved in 60% and some reduction in pain was achieved in 86%. These are superb outcomes. Two-thirds of the patients had steatorrhea in follow-up, half acquired after surgery; 37% developed new-onset diabetes. Again, all these are acceptable rates. Notably, 86% of patients survived 5 years and 65% 10 years, reflecting the known chronic nature of this disease and the likely ongoing ravages of alcoholism. Nutritional improvements were noted in a high percentage of patients independent of their pancreatic function after operation, an observation we have made in the past, and a striking one, I believe.
I have three questions. Can you review with us your formula for choosing among the operations that are available for resection, particularly how you decide between a Frey’s procedure and a Begar procedure?
Number two, you don’t mention narcotics use, and I must say in my huge experience with these patients this overshadows every bit of patient management. I am wondering if you monitor this and whether you have any thoughts on the impact of narcotic dependence on the management of these patients after surgery?
And number three, regarding exocrine insufficiency, there are some mysteries you have exposed. It was shown patients with postop exocrine insufficiency correlated with persistent pain, and your patients with low pre-op BMI had a higher chance of developing exocrine insufficiency and a higher risk of persistent pain. Do you have any thoughts on the connection between this exocrine function and the pain?
I congratulate you and your colleagues on a thorough and superbly analyzed review of these very complex patients.
Dr. Makowiec: Thank you, Dr. Nealon. Your first question was about the choice of the type of operation. After all the experience we have with our patients and our studies, we try to perform the less invasive or the smallest operation possible. That means, in ascending order of complexity, the Frey operation, the Beger operation and PD depending on the anatomy of chronic pancreatitis and the organ complications. For example, patients with pancreatic duct dilatation and a small or medium sized pancreatic head mass will receive a Frey operation. If a patient has a larger mass, a lot of fibrosis in the pancreatic head with concomitant bile duct stenosis, he will undergo Beger’s procedure. In more than 50% of the Beger operations in our series a bilioenteric anastomosis was included, with good results in the vast majority regarding clearance of the bile ducts. If patients have a suspicion of malignancy or an enormous inflammatory mass of the pancreatic head with duodenal destruction, destruction of the antrum of the stomach, we perform a pylorus-preserving pancreaticoduodenectomy.
Regarding the second question, we have no reliable data on the use of narcotics before and after surgery. Preoperatively about one-fourth of the patients took opioids, and three-fourths of the patients had some form of peripheral analgesics. In our follow-up examinations we always asked for the use of narcotics and other analgetics, but I think that the results were not very reliable, again related to the use of alcohol. So we don’t think that the data given by the patients regarding pain medications are scientifically reliable.
Your third question was about the correlation between pain, exocrine insufficiency, and body mass index. We saw that patients with exocrine insufficiency had, more frequently, pain. I think that this is a sign of advanced disease. Another reason is probably, especially patients with severe pain and frequently recurrent pain, the continuous use of alcohol. As for informations about pain medication, this information is also not very reliable. However, during many phone contacts with home physicians, we heard that in most cases with severe or frequent pain these patients continue to drink.
Low body mass index is probably a sign of severe and advanced chronic pancreatitis. These patients are malnourished just because they can’t eat more due to abdominal pain.
Dr. L. Traverso (Seattle, WA): Frank, thank you for showing us all these details in just 10 minutes. I have three questions. When we reviewed our patients after a five-year follow-up following the Whipple operation for chronic pancreatitis those with diabetes preoperatively had better pain relief. Did you see that association?
Number two, we found it valuable to compare the patient’s pre-op pain to their post-op pain and whether they had received some benefit from the operation. With that method we observed every patient indicated they were improved and 76% had complete pain relief.
Third, we all have to be accountable when deciding to resect the head of the pancreas in these patients with documented chronic pancreatitis and chronic abdominal pain. The best way is to make sure they really have severe chronic pancreatitis before resection. The first slide you showed on preoperative ductal anatomy indicated that about 87% or so had large duct disease. From that slide, a little over 10% of your patients could have had a normal pancreatic duct. Was that the case? Did they all have abnormal pancreatic ducts? The method that we used was the Cambridge Classification of Image Severity described by Axon (Axon ATR, Classen M, Cotton PB, et al. Pancreatography in chronic pancreatitis: international definitions. Gut 1984; 25:1107-1112). To qualify for resection a patient had to have the worst case of image severity, i.e., stage IV. The minimal disease required to have that category was a major pancreatic duct stricture in the head, with or without stones, with or without duct dilatation. When pancreatic surgeons are accountable that way then almost every patient after head resection for true chronic pancreatitis will get pain relief, provided they don’t start drinking alcohol again.
Dr. Makowiec: Regarding our data, I can confirm some inverse correlation between the presence of diabetes and pain. We found that the absence of diabetes was a risk factor for pain. I have no explanation for this phenomenon.
Many of the patients who noted to have pain on their questionnaires also noted that they are satisfied with the operation because they had clearly less pain. Regarding pain assessment we had one problem: In 60% of our patients we had no complete preoperative pain documentation regarding frequency or a visual analog scale. So we can hardly compare the data.
About 20 % of our patients had no relevant dilatation of the pancreatic duct. However, most of those had inflammatory masses and/or bile duct stenosis. I, therefore, agree completely with you that pancreatic head resection may be appropriate in these cases, even without large duct disease.
Rights and permissions
About this article
Cite this article
Riediger, H., Adam, U., Fischer, E. et al. Long-term Outcome After Resection for Chronic Pancreatitis in 224 Patients. J Gastrointest Surg 11, 949–960 (2007). https://doi.org/10.1007/s11605-007-0155-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-007-0155-6