Summary
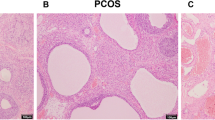
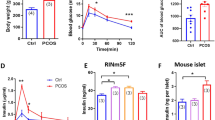
This study investigated the abnormal expression of ATP synthase β-subunit (ATPsyn-β) in pancreas islets of rat model of polycystic ovary syndrome (PCOS) with type 2 diabetes mellitus (T2DM), and the secretion function changes after up-regulation of ATP5b. Sixty female SD rats were divided into three groups randomly and equally. The rat model of PCOS with T2DM was established by free access to the high-carbohydrate/high-fat diet, subcutaneous injections of DHEA, and a single injection of streptozotocin. The pancreas was removed for the detection of the ATPsyn-β expression by immunohistochemical staining, Western blotting and reverse transcription-PCR (RT-PCR). The pancreas islets of the rats were cultured, isolated with collagenase V and purified by gradient centrifugation, and the insulin secretion after treatment with different glucose concentrations was tested. Lentivirus ATP5b was successfully constructed with the vector of GV208 and transfected into the pancreas islets for the over-expression of ATPsyn-β. The insulin secretion and intracellular ATP content were determined after transfection of the PCOS-T2DM pancreas islets with Lenti-ATP5b. The results showed that the expression of ATPsyn-β protein and mRNA was significantly decreased in the pancreas of PCOS-T2DM rats. The ATP content in the pancreas islets was greatly increased and the insulin secretion was improved after the up-regulation of ATPsyn-β in the pancreas islets transfected with lenti-ATP5b. These results indicated that for PCOS, the ATPsyn-β might be one of the key factors for the attack of T2DM.
Similar content being viewed by others
References
Ollila ME, West S, Keinänen-Kiukaanniemi S, et al. Overweight and obese but not normal weight women with PCOS are at increased risk of type 2 diabetes mellitus—a prospective, population-based cohort study. Hum Reprod, 2017,32(2):423–431
Moran LJ, Misso ML, Wild RA, et al. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update, 2010,16(4):347–363
Sim SY, Chin SL, Tan JL, et al. Polycystic ovary syndrome in type 2 diabetes: does it predict a more severe phenotype? Fertil Steril, 2016,106(5):1258–1263
Deugarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril, 2005,83(5):1454–1460
Ashcroft FM, Rorsman P. Diabetes mellitus and the ß cell: The last ten years. Cell, 2012,148(6):1160–1171
Stitzel ML, Kycia I, Kursawe R, et al. Transcriptional regulation of the pancreatic islet: implications for islet function. Curr Diabetes Rep, 2015,15(9):665
Pasquali L, Gaulton KJ, Rodríguez-Seguí SA, et al. Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat Genet, 2014,46(2):136–143
Lawlor N, Khetan S, Ucar D, et al. Genomics of islet (dys)function and type 2 diabetes. Trends Genet, 2017, DOI: http://dx.doi.org/10.1016/j.tig.2017.01.010. [Epub ahead of print] pii:S0168-9525(17)30021-5.
Szendroedi J, Roden M. Mitochondrial fitness and insulin sensitivity in humans. Diabetologia, 2008,51(12):2155–2167
Hahn A, Parey K, Bublitz M, et al. Structure of a complete ATP synthase dimer reveals the molecular basis of inner mitochondrial membrane morphology. Mol Cell, 2016,63(3):445–456
Walker JE. Structure and mechanism of ATP syntha-se. BBA-Bioenergetics, 2016,1587:3
Singh KB. Persistent estrus rat models of polycystic ovary disease: an update. Fertil Steril, 2005,84(Suppl 2):1228–1234
Walters KA, Allan CM. Rodent models for human polycystic ovary syndrome. Biol Reprod, 2012,86(5):1–12
Mannerås L, Cajander S, Holmäng A, et al. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology, 2007,148(8):3781–3791
Shi DN, Vine DF. Animal models of polycystic ovary syndrome: a focused review of rodent models in relationship to clinical phenotypes and cardiometabolic risk. Fertil Steril, 2012,98(1):185–193
Zhai HL, Wu H, Xu H, et al. Trace glucose and lipid metabolism in high androgen and high-fat diet induced polycystic ovary syndrome rats. Reprod Biol Endocrin, 2012,10(1):55
Srinivasan K, Viswanad B, Asrat L, et al. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res, 2005,52(4):313–320
Teede HJ, Hutchison S, Zoungas S. Insulin resistance, the metabolic syndrome, diabetes, and cardiovascular disease risk in women with PCOS. Endocrine, 2006,30(1):45–53
Petersen KF, Dufour S, Shulman GI. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLOS Med, 2005,2(9):879–884
Dong KL, Ni H, Wu ML, et al. ROS-mediated glucose metabolic reprogram induces insulin resistance in type 2 diabetes. Biochem Bioph Res C, 2016,476(4):204–211
Grapengießser E, Dansk H, Hellman B. Pulses of external ATP aid to the synchronization of pancreatic ß-cells by generating premature Ca2+ oscillations. Biochem Pharmacol, 2004,68(4):667–674
Fujimoto S, Nabe K, Takehiro M, et al. Impaired metabolism-secretion coupling in pancreatic beta-cells: role of determinants of mitochondrial ATP production. Diabetes Res Clin Pr, 2007,77(Suppl 1):S2–S10
Wang L, Xu F, Zhang XJ, et al. Effect of high-fat diet on cholesterol metabolism in rats and its association with Na+/K+-ATPase/Src/Perk signaling pathway. J Huazhong Univ Sci Technolog Med Sci, 2015,35(4):490–494
Saleh MC, Fatehi-Hassanabad Z, Wang R, et al. Mutated ATP synthase induces oxidative stress and impaired insulin secretion in beta-cells of female BHE/cdb rats. Diabetes-Metab Res, 2008,24(5):392–403
Pedersen PL. Transport ATPases into the year 2008: a brief overview related to types, structures, functions and roles in health and disease. J Bioenerg Biomembr, 2007,39(5):349–355
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, W., Li, Sj., Yin, Tl. et al. ATP synthase β-subunit abnormality in pancreas islets of rats with polycystic ovary syndrome and type 2 diabetes mellitus. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 37, 210–216 (2017). https://doi.org/10.1007/s11596-017-1717-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-017-1717-9