Summary
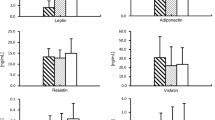
This study examined the adipocytokine-vascular interactions and link between epicardial adipose tissue and coronary artery atherosclerosis. Thirty-four patients undergoing open heart surgery were chosen randomly, and divided into group I (non-coronary artery disease group) and group II (coronary artery disease group). Blood samples were taken through peripheral vein prior to surgery. Plasma levels of a panel of proteins (adiponectin, IL-10, TNF-α) were detected by using ELISA. Epicardial adipose tissue was taken near the proximal tract of the right coronary artery and subcutaneous adipose was taken from the leg before cardiopulmonary bypassing, adiponectin and CD68 + were detected by using RT-PCR and immunohistochemistry. Our results showed that plasma adiponectin level was significantly lower in the group II as compared with group I (P<0.05). There were no differences in plasma concentration (IL-10, TNF-α, tatal-chol, HDL-chol, LDL-chol) between group I and group II. The number of CD68+ cells in epicardial adipose tissue of group II was significantly higher than that in subcutaneous adipose tissue. Adiponectin mRNA expression was 6 fold higher in subcutaneous adipose tissue than in epicardial adipose tissue of group II (P<0.01). Furthermore, the level of adiponectin mRNA in the epicardial adipose tissue in group II was also significantly lower than in group I (P<0.05). We are led to conclude that inflammation that occurs locally in epicardial adipose tissue of CAD contributes to the pathogenesis of coronary artery disease.
Similar content being viewed by others
References
Matsuda M, Shimomura I, Sata M, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem, 2002, 277(40):37487–37491
Moro C, Klimcakova E, Lolmède K, et al. Atrial natriuretic peptide inhibits the production of adipokines and cytokines linked to inflammation and insulin resistance in human subcutaneous adipose tissue. J Diabetologia, 2007,50(5):1038–1047
Tsuchida A, Yamauchi T, Takekawa S, et al. Peroxisome proliferator-activated receptor (PPAR) activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPAR, PPAR, and their combination. J Diabetes, 2005, 54(12):3358–3370
Takaoka M, Suzuki H, Shioda S, et al. Endovascular injury induces rapid phenotypic changes in perivascular adipose tissue. J Arterioscler Thromb Vasc Biol, 2010,30(8):1576–1582
Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, et al. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J, 2009,23(1):241–258
Li S, Shin HJ, Ding EL, et al. Adiponectin levels and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA, 2009,302(2): 179–188
Chen XZ, Sun ZQ, Zheng XY, et al. Adiponectin inhibits free fatty acids -activated Toll-like receptor 4 signaling pathway in monocytes. Chin J Pathophysiol, 2008, 24(11):2117–2120
Nakamura Y, Shimada K, Fukuda D, et al. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. J Heart, 2004,90(5):528–533
Arita Y, Kihara S, Ouchi N, et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. J Circulation. 2002, 105(24):2893–2898
Ouchi N, Kihara S, Arita Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation, 2001,103(8):1057–1063
Chen XZ, Sun ZQ, Du XL, et al. Study on the relationship between heat shock protein 70 and toll-like receptor-4 of monocytes. J Huazhong Univ Sci Technolog [Med Sci], 2004,24(6):560–562
Waki H, Yamauchi T, Kamon J, et al. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. J Endocrinology, 2005,146(2):790–796
Yin X, Tu L, Yang H. Effect of simvastatin on IL-6 and adiponectin secretion and mRNA expression in 3T3-L1 adipocytes. J Huazhong Univ Sci Technolog [Med Sci], 2007,27(3):248–251
Wang TD, Lee WJ, Shih FY, et al. Relations of epicardial adipose tissue measured by multidetector computed tomography to components of the metabolic syndrome are region-specific and independent of anthropometric indexes and intraabdominal visceral fat. J Clin Endocrinol Metab, 2009,94(2):662–669
Wolk R, Berger P, Lennon RJ, et al. Association between plasma adiponectin levels and unstable coronary syndromes. J Eur Heart J, 2007,28(3):292–298
Langheim S, Dreas L, Veschini L, et al. Increased expression and secretion of resistin in epicardial adipose tissue of patients with acute coronary syndrome. J Am J Physiol Heart Circ Physiol, 2010,298(3):H746–H753
Iacobellis G, Ribaudo MC, Assael F, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab, 2003,88(11):5163–5168
Roubícek T, Dolinková M, Bláha J, et al. Increased angiotensinogen production in epicardial adipose tissue during cardiac surgery: possible role in a postoperative insulin resistance. J Physiol Res, 2008,57(6):911–917
Dutour A, Achard V, Sell H, et al. Secretory type II phospholipase A2 is produced and secreted by epicardial adipose tissue and overexpressed in patients with coronary artery disease. J Clin Endocrinol Metab, 2010,95(2): 963–967
Kremen J, Dolinkova M, Krajickova J, et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J Clin Endocrinol Metab, 2006,91(11):4620–4607
Zhou Y, Zhang M, Guo W, et al. Expression of resistin protein in normal human subcutaneous adipose tissue and pregnant women subcutaneous adipose tissue and placenta. J Huazhong Univ Sci Technolog [Med Sci], 2006, 26(3):288–291
Wei G, Zhang M, Mei Y, Dong J. Expression of cytokines IL-2, IL-10 and TNF-alpha in mice with herpes simplex viral encephalitis. J Huazhong Univ Sci Technolog [Med Sci], 2006,26(3):308–310
Salvati VM, Mazzarella G, Gianfrani C, et al. Recombinant human interleukin 10 suppresses gliadin dependent T cell activation in ex vivo cultured coeliac intestinal mucosa. J Gut, 2005,54(1):46–53
Baker AR, Harte AL, Howell N, et al. Epicardial adipose tissue as a source of nuclear factor-kappaB and c-Jun N-terminal kinase mediated inflammation in patients with coronary artery disease. J Clin Endocrinol Metab, 2009, 94(1):261–267
Silaghi A, Achard V, Paulmyer-Lacroix O, et al. Expression of adrenomedullin in human epicardial adipose tissue: role of coronary status. J Am J Physiol Endocrinol Metab, 2007,293(5):E1443–E1450
Gruen ML, Hao M, Piston DW, et al. Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxis. Am J Physiol Cell Physiol, 2007,293(5):C1481–8148
Bumrungpert A, Kalpravidh RW, Chuang CC, et al. Xanthones from mangosteen inhibit inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. J Nutr, 2010,140(4): 842–847
Henrichot E, Juge-Aubry CE, Pernin A, et al. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? J Arterioscler Thromb Vasc Biol. 2005,25(12):2594–2599
Herder C, Baumert J, Thorand B, et al. Chemokines and incident coronary heart disease: results from the MONICA/KORA Augsburg case-cohort study, 1984–2002. J Arterioscler Thromb Vasc Biol, 2006,26(9):2147–2152
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by a grant from the National Natural Science Foundation of China (No. 30872541 C160502), and fund for Doctoral Program of Ministry of Education of China (No. 200804871116).
Rights and permissions
About this article
Cite this article
Chen, X., Jiao, Z., Wang, L. et al. Roles of human epicardial adipose tissue in coronary artery atherosclerosis. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 30, 589–593 (2010). https://doi.org/10.1007/s11596-010-0547-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-010-0547-9