Summary
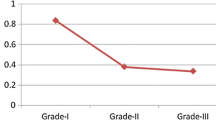
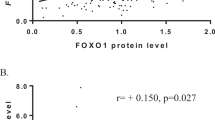
FANCD2 is involved in DNA damage repair and maintenance of chromosome stability. The purpose of this study was to investigate the expression of FANCD2 in sporadic breast cancer tissues and its association with clinicopathological features. A total of 162 Chinese women with invasive breast carcinoma who had no family history in first-degree relatives and 12 normal breast tissues were examined. The expression of FANCD2 was detected by immunohistochemical staining based on a tissue microarray technique. SAS system was used to analyze the data. Twenty-one out of the 162 invasive breast cancers (13%) were negative for FANCD2. The mean percentage of FANCD2 positive cells was significantly lower in breast cancers than in controls (P<0.05). FANCD2 expression was significantly inversely associated with histological grade and TNM stage (P<0.05), but not with axillary lymph node status or other conventional prognostic markers such as ER, PR, Her-2 and PCNA (P>0.05). It was suggested that FANCD2 may play a critical role in breast carcinogenesis. It may become a valuable and independent marker for identifying women with sporadic breast cancer and evaluating the prognosis.
Similar content being viewed by others
References
King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science, 2003,302(5645):643–646
Bordeleau L, Panchal S, Goodwin P. Prognosis of BRCA-associated breast cancer: a summary of evidence. Breast Cancer Res Treat, 2010,119(1):13–24
Oliveira AM, Ross JS, Fletcher JA. Tumor suppressor genes in breast cancer: the gatekeepers and the caretakers. Am J Clin Pathol, 2005,124(Suppl):S16–28
Tsuda H. Gene and chromosomal alterations in sporadic breast cancer: correlation with histopathological features and implications for genesis and progression. Breast Cancer, 2009,16(3):186–201
van der Groep P, Hoelzel M, Buerger H, et al. Loss of expression of FANCD2 protein in sporadic and hereditary breast cancer. Breast Cancer Res Treat, 2008,107(1): 41–47
Youssoufian H. Fanconi anemia and breast cancer: what’s the connection? Nat Genet, 2001,27(4):352–353
Packeisen J, Korsching E, Herbst H, et al. Demystified Tissue microarray technology. Mol Pathol, 2003,56(4): 198–204
Camp RL, Neumeister V, Rimm DL. A decade of tissue microarrays: progress in the discovery and validation of cancer biomarkers. J Clin Oncol, 2008,26(34): 5630–5637
Voduc D, Kenney C, Nielsen TO. Tissue microarrays in clinical oncology. Semin Radiat Oncol, 2008,18(2): 89–97
Vitolo MI, Weiss MB, Szmacinski M, et al. Deletion of PTEN promotes tumorigenic signaling, resistance to anoikis, and altered response to chemotherapeutic agents in human mammary epithelial cells. Cancer Res, 2009,69(21):8275–8283
DiTullio RA Jr, Mochan TA, Venere M, et al. 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nat Cell Biol, 2002,4(12):998–1002
Bartkova J, Horejsí Z, Koed K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature, 2005,434(7035): 864–870
Barroso E, Milne RL, Fernández LP, et al. FANCD2 associated with sporadic breast cancer risk. Carcinogenesis, 2006,27(9):1930–1937
Barroso E, Pita G, Arias JI, et al. The Fanconi anemia family of genes and its correlation with breast cancer susceptibility and breast cancer features. Breast Cancer Res Treat, 2009,118(3):655–660
D’Andrea AD, Grompe M. The Fanconi anemia/BRCA pathway. Nat Rev Cancer, 2003,3(1):23–34
Ohashi A, Zdzienicka MZ, Chen J, et al. Fanconi anemia complementation group D2 (FANCD2) functions independently of BRCA2- and RAD51-associated homologous recombination in response to DNA damage. J Biol Chem, 2005,280(15):14 877–14 883
Shen X, Do H, Li Y, et al. Recruitment of fanconi anemia and breast cancer proteins to DNA damage sites is differentially governed by replication. Mol Cell, 2009,35(5): 716–723
Houghtaling S, Timmers C, Noll M, et al. Epithelial cancer in Fanconi anemia complementation group D2 (Fancd2) knockout mice. Genes Dev, 2003,17(16): 2021–2035
Levitus M, Joenje H, de Winter JP. The Fanconi anemia pathway of genomic maintenance. Cell Oncol, 2006, 28(1–2):3–29
Ling C, Ishiai M, Ali AM, et al. FAAP100 is essential for activation of the Fanconi anemia-associated DNA damage response pathway. EMBO J, 2007,26(8): 2104–2114
Howlett NG, Harney JA, Rego MA, et al. Functional interaction between the Fanconi anemia D2 protein and proliferating cell nuclear antigen (PCNA) via a conserved putative PCNA interaction motif. J Biol Chem, 2009,284(42):28 935–28 942
Garcia-Higuera I, Taniguchi T, Ganesan S, et al. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol Cell, 2001,7(2):249–262
Wang X, Andreassen PR, D’Andrea AD. Functional interaction of monoubiquitinated FANCD2 and BRCA2/FANCD1 in chromatin. Mol Cell Biol, 2004,24(13):5850–5862
Turner N, Tutt A, Ashworth A. Hallmarks of’ BRCAness’ in sporadic cancers. Nat Rev Cancer, 2004, 4(10):814–819
Chirnomas D, Taniguchi T, de la Vega M, et al. Chemosensitization to cisplatin by inhibitors of the Fanconi anemia/BRCA pathway. Mol Cancer Ther, 2005,5(4): 952–961
Lyakhovich A, Surralles J. FANCD2 depletion sensitizes cancer cells repopulation ability in vitro. Cancer Lett, 2007,256(2):186–195
Powell SN, Kachnic LA. Therapeutic exploitation of tumor cell defects in homologous recombination. Anticancer Agents Med Chem, 2008,8(4):448–460
Willers H, Taghian AG, Luo CM, et al. Utility of DNA repair protein foci for the detection of putative BRCA1 pathway defects in breast cancer biopsies. Mol Cancer Res, 2009,7(8):1304–1309
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, B., Chen, R., Lu, J. et al. Expression of FANCD2 in sporadic breast cancer and clinicopathological analysis. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 30, 322–325 (2010). https://doi.org/10.1007/s11596-010-0350-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-010-0350-7