Abstract
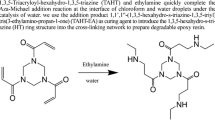
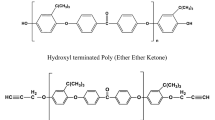
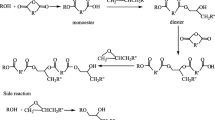
A kind of chemical method that used water as the liquid reaction medium to decompose epoxy resin was studied. The thermosetting epoxy resin was decomposed successfully under the condition of near-critical water. The decomposition rate of epoxy resin raised rapidly as the reaction time and reaction temperature increased. The decomposition reaction products were characterized by infra-red spectra and gas chromatography-mass spectrometry. The phenol, isopropylphenol, 4, 4′-(1-methylethylidene) bis-phenol were found as the main compounds in liquid products, which were common monomers from epoxy resin. When reaction was carried out at the temperature of 260 °C–300 °C, the decomposition mechanism of epoxy resin was envisaged as the ether and ester bonds cracking.
Similar content being viewed by others
References
Takao Masuda, Tatsuhiko Kushino, Toshihiro Matsuda, et al. Chemical Recycling of Mixture of Waste Plastics Using a New Reactor System with Stirred Heat Medium Particles in Steam Atmosphere[J]. Chem. Eng. J., 2001, 82:173–181
Yoshioka T, Keiichi Furukawa, Akitsugu Okuwaki. Chemical Recycling of Rigid-PVC by Oxygen Oxidation in NaOH Solutions at Elevated Temperatures[J]. Polym, Degrad, Stabil., 2000, 67: 285–290
An JY, Bagnell L, Cablewski T, Strauss CR, et al. Applications of High-Temperature Aqueous Media for Synthetic Organic Reactions[J]. J. Org. Chem., 1997, 62: 2 505–2 511
LIU Zhuo-feng, ZENG Jing-cheng, XIAO Jia-yu. Studies on Rheological Behaviors and Processing Windows of Low Viscosity Epoxy Resin for VIMP[J]. J. Wuhan Univ. Technol., 2011, 26(5): 931
Gersifi El, Durand K, Tersac G. Solvolysis of Bisphenol A Diglycidyl Ether/Anhydride Model Networks[J]. Polym Degrad Stabil., 2006, 91(4):690–702
Braun D, Gentzkow1 W von, Rudolf AP. Hydrogenolytic Degradation of Thermosets[J]. Polym. Degrad. Stabil., 2001,74: 25–32
Dang Weirong, Masatoshi Kubouchi, Shurou Yamamoto, et al. An Approach to Chemical Recycling of Epoxy Resin Cured with Amine Using Nitric Acid[J]. Polym., 2002, 43(10): 2 953–2 958
Jinyang Chen, Guiyang Liu. Catalytic Hydrolysis of Waste Nylon 6 to Produce e-Caprolactamin Sub-Critical Water[J]. J. Anal. Appl. Pyrol., 2010, 87:50–55
Liu Yuyan, Li Li and Meng Linghu. The Experimental Research on Recycling of Aramid Fibers by Solvent Method [J]. J. Reinf. Plast. Comp., 2009, 28(18): 2 211–2 220
Bai Yongping, Wang Zhi, Feng Liqun. Chemical Recycling of Carbon Fibers Reinforced Epoxy Resin Compositesin Oxygen in Supercritical Water[J]. Mater. Design, 2010, 31(2): 999–1 002
Raul Pin, Ero-Hernanz, Christopher Dodds, et al. Chemical Recycling of Carbon Fibre Reinforced Composites in Near-Critical and Supercritical Water[J]. Compos. Part A-Appls., 2008, 39(3): 454–461
Motonobu Goto. Chemical Recycling of Plastics Using Sub- and Supercritical Fluids[J]. J. Supercrit Fluid, 2009, 47: 500–507
Yoshiki Satoa, Yasuhiko Kondob, Koji Tsujitac, et al. Degradation Behaviour and Recovery of Bisphenol-A from Epoxy Resin and Polycarbonate Resin by Liquid-Phase Chemical Recycling[J]. Polym. Degrad. Stabil., 2005, 89(2): 317–326
Seok-Ho Lee, Hwan-Oh Choi, Jung-Seok Kim, et al. Circulating Flow Reactor for Recycling of Carbon Fiber from Carbon Fiber Reinforced Epoxy Composite[J]. Korean J. Chem. Eng., 2011, 28(1): 449–454
Brunner GJ. Near and Supercritical Water. Part II: Oxidative Processes[J]. J. Supercrit Fluid. 2009, 47: 373–381
Amelia Torres, Isabel de Marco, Blanca M. Caballero. GC-MS Analysis of the Liquid Products Obtained in the Pyrolysis of Fibre-Glass Polyester Sheet Moulding Compound[J]. J. Anal. Appl. Pyrol., 2000, 58–59(3):189–203
Ali Karaduman, Emir H Simsek, Burhanettin C, et al. Thermal Degradation of Polystyrene Wastes in Various Solvents[J]. J. Anal. Appl. Pyrol., 2002, 62: 273–280
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by the National Natural Science Foundation of China (No. 50973023) and the Program for New Century Excellent Talents in University(No. NCET-09-0060)
Rights and permissions
About this article
Cite this article
Gong, X., Liu, Y., Jia, X. et al. Decomposition behavior and decomposition products of epoxy resin cured with MeHHPA in near-critical water. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 28, 781–786 (2013). https://doi.org/10.1007/s11595-013-0768-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-013-0768-4