Abstract
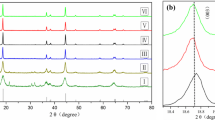
The layered LiNi0.6Co0.2−x Mn0.2Mg x O2 (x=0.00, 0.03, 0.05, 0.07) cathode materials were prepared by a co-precipitation method. The properties of the Mg-doped LiNi0.6Co0.2Mn0.2O2 were investigated by X-ray diffraction (XRD), scanning electron microscopy (SEM), and electrochemical measurements. XRD studies showed that the Mg-doped LiNi0.6Co0.2Mn0.2O2 had the same layered structure as the undoped LiNi0.6Co0.2Mn0.2O2. The SEM images exhibited that the particle size of Mg-doped LiNi0.6Co0.2Mn0.2O2 was finer than that of the undoped LiNi0.6Co0.2 Mn0.2O2 and that the smallest particle size is only about 1 μm. The Mg-doped LiNi0.6Co0.2Mn0.2O2 samples were investigated on the Li extraction/insertion performances through charge/discharge, cyclic voltammogram (CV), and electrochemical impedance spectra(EIS). The optimal doping content of Mg was that x= 0.03 in the LiNi0.6Co0.2−x Mn0.2Mg x O2 samples to achieve high discharge capacity and good cyclic stability. The electrode reaction reversibility and electronic conductivity was enhanced, and the charge transfer resistance was decreased through Mg-doping. The improved electrochemical performances of the Mg-doped LiNi0.6Co0.2Mn0.2O2 cathode materials are attributed to the addition of Mg2+ ion by stabilizing the layer structure.
Similar content being viewed by others
References
Koksbang R, Barker J, Shi H. Cathode Materials for Lithium Rocking Chair Batteries[J]. Solid State Ionics, 1996, 84: 1–21
Randolph A L, Marcus J P, Esther S T, et al. A Study of the Overcharge Reaction of Lithium-ion Batteries[J]. J. Power Sources, 2001, 97–98: 681
Won-Sub Yoon, Kwang-Bum Kim. Synthesis of LiCoO2 Using Acrylic Acid and Its Electrochemical Properties for Li Secondary Batteries[J]. Journal of Power Source, 1999, 81–82: 517–523
Arai H, Okada S, Sakurai Y, et al. Reversibility of LiNiO2 Cathode[J]. Solid State Ion., 1997, 95(3–4): 275–282
Zhong Sheng-wen, Zhao Yu-juan, LIAN Fang, et al. Characteristics and Electrochemical Performance of Cathode Material Co-coated LiNiO2 for Li-ion Batteries[J]. Trans. Nonferrous Met. SOC. China, 2006, 16: 137–141
Ceder G, Mishra S K. The Stability of Orthorhombic and Monoclinic-layered LiMnO2[J]. Electrochem. Solid-State Lett., 1999, 2(11): 550–552
Koyama Y, Tanaka, I, Adachi H, et al. Crystal and Electronic Structures of Superstructural Li1−x [Co1/3Ni1/3Mn1/3]O2 (0≤x≤1)[J]. J. Power Sources, 2003, 119–121: 644–648
Liu Jingjing, Qiu Weihua, Yu Lingyan, et al. Comparative Experiment on Layered LiMn1/3 Co1/3 Ni1/3O2 as the Alternative Material for LiCoO2[J]. J. University of Science and Technology Beijing, 2007, 14(2): 173
Lee M H, Kang A Y J, Myung S T, et al. Synthetic Optimization of via Co-precipitation[J]. Electroimica Acta, 2004, 50: 939–948
Tsai Y W, Hwang B J, Ceder G, et al. In-situ X-ray Absorption Spectroscopic Study on Variation of LiMn1/3Co1/3Ni1/3O2 Cathode Material during Electrochemical cycling[J]. Chem. Mater., 2005, 17: 3191–3199
Choi J, Manthiram A. Comparison of the Electrochemical Behaviors of Stoichiometric LiMn1/3Co1/3Ni1/3O2 and lithium Excess Li1.03 (Ni1/3Co1/3Mn1/3)0.97O2[J]. Electrochem Solid State Lett., 2004, 7(10): A 365–A 368
Naoaki Y, Tsutomu O. Novel Lithium Insertion Material of LiMn1/3Co1/3Ni1/3O2 for Advanced Lithiumion Batteries[J]. J. Power Sources, 2003, 119–121: 171
Cao H, Zhang Y, Zhang J, Xia BJ, Synthesis and Electrochemical Characteristics of Layered LiNi0.6Co0.2 Mn0.2O2 Cathode Material for Lithium Ion Batteries[J]. Solid State Ionics, 2005, (176): 1207
Yao Zhang, Hui Cao, Jian Zhang, Baojia Xia. Synthesis of LiNi0.6Co0.2Mn0.2O2 Cathode Material by a Carbonate Co-precipitation Method and Its Electrochemical Characterization[ J]. Solid State Ionics, 2006, 177(37–38): 3303–3307
Ambrose R, Nichols Jr, James H Walton. The Autoxidation of Manganous Hydroxide[J]. J. Am. Chem. Soc., 1942, 64(8): 1866–1870
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by the Scientific Research Fund of Hunan Education Department (10C0294)
Rights and permissions
About this article
Cite this article
Fu, C., Zhou, Z., Liu, Y. et al. Synthesis and electrochemical properties of Mg-doped LiNi0.6Co0.2Mn0.2O2 cathode materials for Li-ion battery. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 26, 211–215 (2011). https://doi.org/10.1007/s11595-011-0199-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-011-0199-z