Abstract
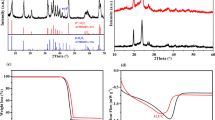
A new series of proton-conducting solid polymer electrolytes with different compositions, comprising polyvinylpyrrolidone (PVPK40) and polymethylmethacrylate (PMMA) as host polymers, methanesulfonic acid (MSA) as a proton-conducting salt, and alumina (Al2O3) as the nanofiller, were prepared using solution casting. High proton-conducting samples were identified and utilized for the construction of primary proton batteries. The electrical properties of the prepared electrolytes were investigated through AC impedance analysis. The highest proton conductivity (2.51 × 10−5 S/cm) was achieved at room temperature by PMMA-PVPK40-MSA-based blended polymer electrolytes (BS3). The discharge characteristics of filler-dispersed solid polymer electrolytes were better than those of other solid polymer electrolytes. The estimated energy density of the constructed proton battery using solid polymer electrolytes with blended polymers and nanofillers was 0.66 and 3.25 Wh kg−1, respectively.










Similar content being viewed by others
References
Xu H, Fathipour S, Kinder EW, Seabaugh AC, Fullerton-Shirey SK (2015) Reconfigurable ion gating of 2H-MoTe2 field-effect transistors using poly (ethylene oxide)-CsClO4 solid polymer electrolyte. ACS Nano 9(5):4900–4910
Lin D, Liu W, Liu Y, Lee HR, Hsu P-C, Liu K, Cui Y (2015) High ionic conductivity of composite solid polymer electrolyte via in situ synthesis of monodispersed SiO2 nanospheres in poly (ethylene oxide). Nano Lett 16(1):459–465
Karuppasamy K, Reddy PA, Srinivas G, Tewari A, Sharma R, Shajan XS, Gupta D (2016) Electrochemical and cycling performances of novel nonafluorobutanesulfonate (nonaflate) ionic liquid based ternary gel polymer electrolyte membranes for rechargeable lithium ion batteries. J Membr Sci 514:350–357
Alwin S, Shajan XS, Karuppasamy K, Warrier K (2017) Microwave assisted synthesis of high surface area TiO2 aerogels: a competent photoanode material for quasi-solid dye-sensitized solar cells. Mater Chem Phys 196:37–44
Karuppasamy K, Kim H-S, Kim D, Vikraman D, Prasanna K, Kathalingam A, Sharma R, Rhee HW (2017) An enhanced electrochemical and cycling properties of novel boronic ionic liquid based ternary gel polymer electrolytes for rechargeable Li/LiCoO2 cells. Sci Rep 7(1):11103 (1–11103 11
Ambika C, Hirankumar G (2016) Characterization of CH3SO3H-doped PMMA/PVP blend-based proton-conducting polymer electrolytes and its application in primary battery. Appl Phys A Mater Sci Process 122(2):113
Gao H, Lian K (2014) Proton-conducting polymer electrolytes and their applications in solid supercapacitors: a review. RSC Adv 4(62):33091–33113
Samsudin A, Lai H, Isa M (2014) Biopolymer materials based carboxymethyl cellulose as a proton conducting biopolymer electrolyte for application in rechargeable proton battery. Electrochim Acta 129:1–13
Guitton J, Dongui B, Mosdale R, Forestier M (1988) New negative metallic electrode for solid batteries with a solid protonic conductor (SPC) as electrolyte. Solid State Ionics 28:847–852
Aslan A, Bozkurt A (2012) Preparation of proton conducting membranes containing bifunctional titania nanoparticles. In: Nanotechnology for sustainable development. Springer, Cham, pp 235–243
Aziz SB, Rasheed MA, Abidin ZH (2017) Optical and electrical characteristics of silver ion conducting nanocomposite solid polymer electrolytes based on chitosan. J Electron Mater 46(10):6119–6130
Sengwa R, Dhatarwal P, Choudhary S (2015) Effects of plasticizer and nanofiller on the dielectric dispersion and relaxation behaviour of polymer blend based solid polymer electrolytes. Curr Appl Phys 15(2):135–143
Ahmad NHB, Isa MINBM (2015) Proton conducting solid polymer electrolytes based carboxymethyl cellulose doped ammonium chloride: ionic conductivity and transport studies. Int J Plast Technol 19(1):47–55
Prajapati G, Roshan R, Gupta P (2010) Effect of plasticizer on ionic transport and dielectric properties of PVA–H3PO4 proton conducting polymeric electrolytes. J Phys Chem Solids 71(12):1717–1723
Malavasi L, Fisher CA, Islam MS (2010) Oxide-ion and proton conducting electrolyte materials for clean energy applications: structural and mechanistic features. Chem Soc Rev 39(11):4370–4387
Gao H, Lian K (2010) Characterizations of proton conducting polymer electrolytes for electrochemical capacitors. Electrochim Acta 56(1):122–127
Shukur M, Ithnin R, Illias H, Kadir M (2013) Proton conducting polymer electrolyte based on plasticized chitosan–PEO blend and application in electrochemical devices. Opt Mater 35(10):1834–1841
Nueangnoraj K, Tomai T, Nishihara H, Kyotani T, Honma I (2016) An organic proton battery employing two redox-active quinones trapped within the nanochannels of zeolite-templated carbon. Carbon 107:831–836
Moniha V, Alagar M, Selvasekarapandian S, Sundaresan B, Boopathi G (2018) Conductive bio-polymer electrolyte iota-carrageenan with ammonium nitrate for application in electrochemical devices. J Non-Cryst Solids 481:424–434
Premalatha M, Mathavan T, Selvasekarapandian S, Selvalakshmi S, Monisha S (2017) Incorporation of NH4Br in tamarind seed polysaccharide biopolymer and its potential use in electrochemical energy storage devices. Org Electron 50:418–425
Manjuladevi R, Thamilselvan M, Selvasekarapandian S, Mangalam R, Premalatha M, Monisha S (2017) Mg-ion conducting blend polymer electrolyte based on poly (vinyl alcohol)-poly (acrylonitrile) with magnesium perchlorate. Solid State Ionics 308:90–100
Premalatha M, Mathavan T, Selvasekarapandian S, Monisha S, Pandi DV, Selvalakshmi S (2016) Investigations on proton conducting biopolymer membranes based on tamarind seed polysaccharide incorporated with ammonium thiocyanate. J Non-Cryst Solids 453:131–140
Karaman B, Bozkurt A (2018) Enhanced performance of supercapacitor based on boric acid doped PVA-H2SO4 gel polymer electrolyte system. Int J Hydrog Energy 43(12):6229–6237
Ramya C, Selvasekarapandian S, Savitha T, Hirankumar G, Baskaran R, Bhuvaneswari M, Angelo P (2006) Conductivity and thermal behavior of proton conducting polymer electrolyte based on poly (N-vinyl pyrrolidone). Eur Polym J 42(10):2672–2677
Ravi M, Kumar KK, Mohan VM, Rao VN (2014) Effect of nano TiO2 filler on the structural and electrical properties of PVP based polymer electrolyte films. Polym Test 33:152–160
Qiao J, Fu J, Lin R, Ma J, Liu J (2010) Alkaline solid polymer electrolyte membranes based on structurally modified PVA/PVP with improved alkali stability. Polymer 51(21):4850–4859
Karuppasamy K, Antony R, Thanikaikarasan S, Balakumar S, Shajan XS (2013) Combined effect of nanochitosan and succinonitrile on structural, mechanical, thermal, and electrochemical properties of plasticized nanocomposite polymer electrolytes (PNCPE) for lithium batteries. Ionics 19(5):747–755
Karuppasamy K, Kim D, Kang YH, Prasanna K, Rhee HW (2017) Improved electrochemical, mechanical and transport properties of novel lithium bisnonafluoro-1-butanesulfonimidate (LiBNFSI) based solid polymer electrolytes for rechargeable lithium ion batteries. J Ind Eng Chem 52:224–234
Karuppasamy K, Reddy PA, Srinivas G, Sharma R, Tewari A, Kumar GH, Gupta D (2017) An efficient way to achieve high ionic conductivity and electrochemical stability of safer nonaflate anion-based ionic liquid gel polymer electrolytes (ILGPEs) for rechargeable lithium ion batteries. J Solid State Electrochem 21(4):1145–1155
Karuppasamy K, Thanikaikarasan S, Antony R, Balakumar S, Shajan XS (2012) Effect of nanochitosan on electrochemical, interfacial and thermal properties of composite solid polymer electrolytes. Ionics 18(8):737–745
Ramesh S, Bing KN (2012) Conductivity, mechanical and thermal studies on poly (methyl methacrylate)-based polymer electrolytes complexed with lithium tetraborate and propylene carbonate. J Mater Eng Perform 21(1):89–94
Sharma JP, Sekhon S (2006) PMMA-based polymer gel electrolytes containing NH4PF6: role of molecular weight of polymer. Mater Sci Eng B 129(1–3):104–108
Ramya C, Selvasekarapandian S, Savitha T, Hirankumar G, Angelo P (2007) Vibrational and impedance spectroscopic study on PVP–NH4SCN based polymer electrolytes. Physica B 393(1–2):11–17
Ramya C, Selvasekarapandian S, Savitha T (2008) Proton-conducting membranes: poly (N-vinyl pyrrolidone) complexes with various ammonium salts. J Solid State Electrochem 12(7–8):807–814
Vijaya N, Selvasekarapandian S, Hirankumar G, Karthikeyan S, Nithya H, Ramya C, Prabu M (2012) Structural, vibrational, thermal, and conductivity studies on proton-conducting polymer electrolyte based on poly (N-vinylpyrrolidone). Ionics 18(1–2):91–99
Pandey K, Lakshmi N, Chandra S (1998) A rechargeable solid-state proton battery with an intercalating cathode and an anode containing a hydrogen-storage material. J Power Sources 76(1):116–123
Rajeswari N, Selvasekarapandian S, Karthikeyan S, Nithya H, Sanjeeviraja C (2012) Lithium ion conducting polymer electrolyte based on poly (vinyl alcohol)–poly (vinyl pyrrolidone) blend with LiClO4. Int J Polym Mater 61(14):1164–1175
Hema M, Selvasekarapandian S, Hirankumar G, Sakunthala A, Arunkumar D, Nithya H (2010) Laser Raman and ac impedance spectroscopic studies of PVA: NH4NO3 polymer electrolyte. Spectrochim Acta A 75(1):474–478
Mahendran O, Rajendran S (2003) Ionic conductivity studies in PMMA/PVdF polymer blend electrolyte with lithium salts. Ionics 9(3–4):282–288
Ambika C, Karuppasamy K, Dhanasekaran V, Lee JY, Regu T, Ajith T, Prasanna K, Kim HS (2018) Effect of dimethyl carbonate (DMC) on the electrochemical and cycling properties of solid polymer electrolytes (PVP-MSA) and its application for proton batteries. Solid State Ionics 321(8):106–114
Dey A, Karan S, De S (2013) Effect of nanoadditives on ionic conductivity of solid polymer electrolyte. Indian J Pure Appl Phys 51(5):281–288
Karuppasamy K, Kim D, Kang YH, Prasanna K, Rhee HW (2017) Improved electrochemical, mechanical and transport properties of novel lithium bisnonafluoro-1-butanesulfonimidate (LiBNFSI) based solid polymer electrolytes for rechargeable lithium ion batteries. J Ind Eng Chem 52(8):224–234
Karuppasamy K, Prasanna K, Kim D, Kang YH, Rhee HW (2017) Headway in rhodanide anion based ternary gel polymer electrolytes (TILGPEs) for applications in rechargeable lithium ion batteries: an efficient route to achieve high electrochemical and cycling performances. RSC Adv 7:19211–19222
Karuppasamy K, Vani CV, Antony R, Balakumar S, Shajan XS (2013) Effect of succinonitrile and nano-hydroxyapatite on ionic conductivity and interfacial stability of polyether-based plasticized nanocomposite polymer electrolytes (PNCSPE). Polym Bull 70(9):2531–2545
Stephan AM, Nahm K (2006) Review on composite polymer electrolytes for lithium batteries. Polymer 47(16):5952–5964
Karthikprabhu S, Karuppasamy K, Dhansekaran V, Prasanna K, Maiyalagan T, Nichelson A, Kathalingam A, Kim HS (2018) Electrochemical performances of LiNi1−xMnxPO4 (x = 0.05–0.2) olivine cathode materials for high voltage rechargeable lithium ion batteries. Appl Surf Sci 449:435–444
Bansod S, Bhoga S, Singh K, Tiwari R (2007) The role of electrolyte in governing the performance of protonic solid state battery. Ionics 13(5):329–332
Kadir M, Majid S, Arof A (2010) Plasticized chitosan–PVA blend polymer electrolyte based proton battery. Electrochim Acta 55(4):1475–1482
Ng L, Mohamad A (2006) Protonic battery based on a plasticized chitosan-NH4NO3 solid polymer electrolyte. J Power Sources 163(1):382–385
Boroglu M, Cavus S, Boz I, Ata A (2011) Synthesis and characterization of poly (vinyl alcohol) proton exchange membranes modified with 4, 4-diaminodiphenylether-2, 2-disulfonic acid. Express Polym Lett 5(5):470–478
Liu H, Ye H, Lin T, Zhou T (2008) Synthesis and characterization of PMMA/Al2O3 composite particles by in situ emulsion polymerization. Particuology 6(3):207–213
Saroj A, Singh R, Chandra S (2014) Thermal, vibrational, and dielectric studies on PVP/LiBF4+ ionic liquid [EMIM][BF4]-based polymer electrolyte films. J Phys Chem Solids 75(7):849–857
Funding
This work was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20194030202320) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2017R1D1A1A09000823).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Regu, T., Ambika, C., Karuppasamy, K. et al. Al2O3-incorporated proton-conducting solid polymer electrolytes for electrochemical devices: a proficient method to achieve high electrochemical performance. Ionics 25, 5117–5129 (2019). https://doi.org/10.1007/s11581-019-03075-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03075-5