Abstract
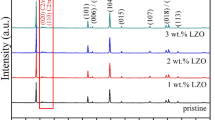
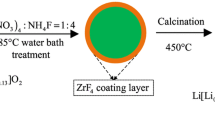
The LaF3-coated Li[Li0.2Mn0.54Ni0.13Co0.13]O2 nanoparticles were synthesized via co-precipitation method followed by simple chemical deposition process. The crystal structure, particle morphology, and electrochemical properties of the bare and coated materials were studied by XRD, SEM, TEM, charge–discharge tests. The results showed that the surface coating on Li[Li0.2Mn0.54Ni0.13Co0.13]O2 nanoparticles were amorphous LaF3 layer with a thickness of about 10–30 nm. After the surface modification with LaF3 films, the coating layer served as a protective layer to suppress the side reaction between the positive electrode and electrolyte, and the Li[Li0.2Mn0.54Ni0.13Co0.13]O2 oxide demonstrated the improved electrochemical properties. The LaF3-coated Li[Li0.2Mn0.54Ni0.13Co0.13]O2 electrode delivered the capacities of 270.5, 247.9, 197.1, 170.0, 142.7, and 109.5 mAh g−1 at current rates of 0.1, 0.2, 0.5, 1, 2, and 5 C rate, respectively. Besides, the capacity retention was increased from 85.1 to 94.8 % after 100 cycles at 0.5 C rate. It implied surface modification with LaF3 played an important role to improve the cyclic stability and rate capacity of the Li-rich nickel manganese oxides.









Similar content being viewed by others
References
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22:587–603
Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D (2011) Challenges in the development of advanced Li-ion batteries: a review. Energ Environ Sci 4:3243–3262
Tang W, Liu LL, Tian S, Li L, Yue YB, Wu YP, Guan SY, Zhu K (2010) Nano-LiCoO2 as cathode material of large capacity and high rate capability for aqueous rechargeable lithium batteries. Electrochem Commun 12:1524–1526
Liu YY, Cao CB, Li J (2010) Enhanced electrochemical performance of carbon nanospheres–LiFePO4 composite by PEG based sol–gel synthesis. Electrochim Acta 55:3921–3926
Zhao S, Bai Y, Chang QJ, Yang YQ, Zhang WF (2013) Surface modification of spinel LiMn2O4 with FeF3 for lithium ion batteries. Electrochim Acta 108:727–735
Xi LJ, Cao CW, Ma RG, Wang Y, Yang SL, Deng JQ, Gao M, Lian F, Lu ZG, Chung CY (2013) Layered Li2MnO3·3LiNi(0.5-x)Mn(0.5-x)Co(2x)O2 microspheres with Mn-rich cores as high performance cathode materials for lithium ion batteries. Phys Chem Chem Phys 15:16579–16585
Du CQ, Zhang F, Ma CX, Wu JW, Tang ZY, Zhang XH, Qu DY (2016) Synthesis and electrochemical properties of Li1.2Mn0.54Ni0.13Co0.13O2 cathode material for lithium-ion battery. Ionics 22:209–218
Thackaray MM, Johnson CS, Vaughey JT, Li N, Hackney SA (2005) Advances in manganese-oxide ‘composite’ electrodes for lithium-ion batteries. J Mater Chem 15:2257–2267
Jin X, Xu QJ, Liu HM, Yuan XL, Xia YY (2014) Excellent rate capability of Mg doped Li[Li0.2Ni0.13Co0.13Mn0.54]O2 cathode material for lithium-ion battery. Electrochim Acta 136:19–26
Zheng JM, Wu XB, Yang Y (2013) Improved electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material by fluorine incorporation. Electrochim Acta 105:200–208
Han ES, Li YP, Zhu LZ, Zhao L (2014) The effect of MgO coating on Li1.17Mn0.48Ni0.23Co0.12O2 cathode material for lithium ion batteries. Solid State Ionics 255:113–119
Shi SJ, Tu JP, Zhang YJ, Zhang YD, Zhao XY, Wang XL, Gu CD (2013) Effect of Sm2O3 modification on Li[Li0.2Mn0.56Ni0.16Co0.08]O2 cathodematerial for lithium ion batteries. Electrochim Acta 108:441–448
Lee SH, Koo BK, Kim JC, Kim KM (2008) Effect of Co3(PO4)2 coating on Li[Co0.1Ni0.15Li0.2Mn0.55]O2 cathode material for lithium rechargeable batteries. J Power Sources 184:276–283
Wang ZY, Liu EZ, He CN, Shi CS, Li JJ, Zhao NQ (2013) Effect of amorphous FePO4 coating on structure and electrochemical performance of Li1.2Ni0.13Co0.13Mn0.54O2 as cathode material for Li-ion batteries. J Power Sources 236:25–32
Jo CH, Cho DH, Noh HJ, Yashiro H, Sun YK, Myung ST (2015) An effective method to reduce residual lithium compounds on Ni-rich Li[Ni0.6Co0.2Mn0.2]O2 active material using a phosphoric acid derived Li3PO4 nanolayer. Nano Res 8:1464–1479
Li LJ, Chen ZY, Zhang QB, Xu M, Zhou X, Zhu HL, Zhang KL (2015) A hydrolysis-hydrothermal route for the synthesis of ultrathin LiAlO2-inlaid LiNi0.5Co0.2Mn0.3O2 as a high-performance cathode material for lithium ion batteries. J Mater Chem A 3:894–904
Zheng JM, Zhang ZR, Wu XB, Dong ZX, Zhu Z, Yang Y (2008) The effects of AlF3 coating on the performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 positive electrode material for lithium-ion battery. J Electrochem Soc 155:A775–A782
Sun SW, Wan N, Wu Q, Zhang XP, Pan D, Bai Y, Lu X (2015) Surface-modifiedLi[Li0.2Ni0.17Co0.07Mn0.56]O2 nanoparticles with MgF2 as cathode for Li-ion battery. Solid State Ionics 278:85–90
Kang SF, Qin HF, Fang Y, Li X, Wang YG (2014) Preparation and electrochemical performance of Yttriumdoped Li[Li0.20Mn0.534Ni0.133Co0.133]O2 as cathode material for lithium-ion batteries. Electrochim Acta 144:22–30
Li N, An R, Su YF, Wu F, Bao LY, Chen L, Zheng Y, Shou HF, Chen S (2013) The role of yttrium content in improving electrochemical performance of layered lithium-rich cathode materials for Li-ion batteries. J Mater Chem A1:9760–9767
Li A, Chen Y, Chen H, Shu D, Li W, Wang H, Dou C, Zhang W, Chen S (2009) Electrochemical behavior and application of lead–lanthanum alloys for positive grids of lead-acid batteries. J Power Sources 189:1204–1211
Mohan P, Kalaignan GP (2013) Electrochemical performances of co-substituted (La and Li) LiLax_yLiyNi1_xO2 cathode materials for rechargeable lithium-ion batteries. Mater Res Bull 48:3049–3057
Ghanty C, Basu RN, Majumder SB (2014) Electrochemical performances of 0.9Li2MnO3–0.1Li(Mn0.375Ni0.375Co0.25)O2 cathodes: role of the cycling induced layered to spinel phase transformation. Solid State Ionics 256:19–28
Chen M, Chen DR, Liao YH, Huang QM, Li WS (2015) Influence of Fe substitution on cycling stability of Li[Li0.2Ni0.13Mn0.54Co0.13]O2 cathode for lithium ion batteries. Ionics 21:1827–1833
Karino W (2016) Order of the transition metal layer in LiNi1/3Co1/3Mn1/3O2 and stability of the crystal structure. Ionics 22:991–995
Shi SJ, Tu JP, Mai YJ, Zhang YQ, Tang YY, Wang XL (2012) Structure and electrochemical performance of CaF2 coated LiMn1/3Ni1/3Co1/3O2 cathode material for Li-ion batteries. Electrochim Acta 83:105–112
Deng H, Belharouak I, Wu H, Dambournet D, Amine KDambournet D, Amine K, (2010) Effect of cobalt incorporation and lithium enrichment in lithium nickel manganese oxides. J Electrochem Soc 157:A776–A781
Yu DYW, Katsunori Y, Yoshio K, Hiroshi N (2009) Electrochemical activities in Li2MnO3. J Electrochem Soc 156:A417–A424
Yabuuchi N, Yoshii K, Myung ST, Nakai I, Komaba S (2011) Detailed Studies of a high-capacity electrode material for rechargeable batteries, Li2MnO3−LiCo1/3Ni1/3Mn1/3O2. J Am Chem Soc 133:4404–4419
He W, Qian J, Cao Y, Ai X, Yang H (2012) Improved electrochemical performances of nanocrystalline Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material for Li-ion batteries. RSC Adv 2:3423–3429
Liu XY, Liu JL, Huang T, Yu AS (2013) CaF2-coated Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials for Li-ion batteries. Electrochim Acta 109:52–58
Lu C, Wu H, Zhang Y, Liu H, Chen BJ, Wu NT, Wang S (2014) Cerium fluoride coated layered oxide Li1.2Mn0.54Ni0.13Co0.13O2 as cathode materials with improved electrochemical performance for lithium ion batteries. J Power Sources 267:682–691
Croy JR, Kim D, Balasubramanian M, Gallagher K, Kang SH, Thackeray MM (2012) Countering the voltage decay in high capacity xLi2MnO3•(1–x)LiMO2 electrodes (M=Mn, Ni, Co) for Li-Ion batteries. J Electrochem Soc 159:A781–A790
Liu J, Jayan BR, Manthiram A (2010) Conductive surface modification with aluminum of high capacity layered Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathodes. J Phys Chem C 114:9528–9533
Thackeray MM, Kang SH, Johnson CS, Vaughey JT, Benedek R, Hackney SA (2007) Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J Mater Chem 17:3112–3125
Amalraj F, Talianker M, Markovsky B, Sharon D, Burlaka L, Shafir G, Zinigrad E, Haik O, Aurbach D, Lampert J, Dobrick MS, Garsuch A (2013) Studies of Li and Mn-Rich Lix[MnNiCo]O2 electrodes: electrochemical performance, structure, and the effect of the aluminum fluoride coating batteries and energy storage. J Electrochem Soc 160:A324–A337
Sun YK, Lee MJ, Yoon CS, Hassoun J, Amine K, Scrosati B (2012) The role of AlF3 coatings in improving electrochemical cycling of Li-enriched nickel-manganese oxide electrodes for Li-Ion batteries. Adv Mater 24:1192–1196
Park SH, Kang SH, Johnson CS, Amine K, Thackeray MM (2007) Lithium–manganese–nickel-oxide electrodes with integrated layered–spinel structures for lithium batteries. Electrochem Commun 9:262–268
Acknowledgments
This work is financially supported by the National Natural Science Foundation of China (51405419).
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Lanthanum Fluoride is used as the coating material.
• LaF3-coated Li[Li0.2Mn0.54Ni0.13Co0.13]O2 is synthesized successfully.
• The discharge capacity increases from 89.2 to 109.5 mAh g−1 at 5 C rate after LaF3 coating.
• The 100-cycle capacity retention is improved from 85.1 to 94.8 % after LaF3 coating.
• The LaF3 films restrain the side reaction between positive electrode and electrolyte during cycling.
Rights and permissions
About this article
Cite this article
Li, CD., Yao, ZL., Xu, J. et al. Surface-modified Li[Li0.2Mn0.54Ni0.13Co0.13]O2 nanoparticles with LaF3 as cathode for Li-ion battery. Ionics 23, 549–558 (2017). https://doi.org/10.1007/s11581-016-1823-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1823-x