Abstract
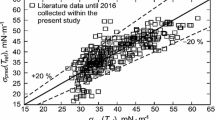
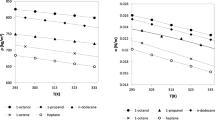
The group method of data handling (GMDH) was utilized to estimate the surface tension of 59 ionic liquids. In this regard, an extensive experimental data was selected from literature over the range 268.3 to 532.4 K. The GMDH model can predict the surface tension of ionic liquids by a grand polynomial correlation function of molar density, reduced boiling temperature, reduced temperature and pressure, acentric factor, and critical compressibility factor. The values of the GMDH model showed a very good regression with the experimental data. The average absolute relative deviation (AARD%) of the GMDH model for all ionic liquids was 4.59 % which indicated a good precision in comparison with those obtained from the generalized dimensionless equation, Mousazadeh–Faramarzi equation, and Parachor equation with AARDs% of 10.31, 13.02, and 11.89, respectively.




Similar content being viewed by others
Abbreviations
- IL:
-
Ionic liquid
- [C2OHmim] [BF4]:
-
1-(2-Hydroxyethyl)-3-methylimidazolium tetrafluoroborate
- [C10C10im] [bti]:
-
1,3-Decylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [C4C4im] [bti]:
-
1,3-Dibutylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [C2C2im] [bti]:
-
1,3-Diethylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [C1C1im] [bti]:
-
1,3-Dimethylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [Dmim] [MSO4]:
-
1,3-Dimethylimidazolium methylsulfate
- [C3C3im] [bti]:
-
1,3-Dipropylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [C7C7im] [bti]:
-
1,3-Heptylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [C6C6im] [bti]:
-
1,3-Hexylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [C9C9im] [bti]:
-
1,3-Nonylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [C8C8im] [bti]:
-
1,3-Octylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [C5C5im] [bti]:
-
1,3-Pentylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [Bmpyr] [bti]:
-
1-Butyl-1-methylpyrrolidinium bis[(trifluoromethyl)sulfonyl]imide
- [Bdmim] [bti]:
-
1-Butyl-2,3-dimethylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [Bdmim] [PF6]:
-
1-Butyl-2,3-dimethylimidazolium hexafluorophosphate
- [Bmim] [bti]:
-
1-Butyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [Bmim] [Cl]:
-
1-Butyl-3-methylimidazolium chloride
- [Bmim] [dca]:
-
1-Butyl-3-methylimidazolium dicyanamide
- [Bmim] [PF6]:
-
1-Butyl-3-methylimidazolium hexafluorophosphate
- [Bmim] [I]:
-
1-Butyl-3-methylimidazolium iodide
- [Bmim] [MSO4]:
-
1-Butyl-3-methylimidazolium methylsulfate
- [Bmim] [BF4]:
-
1-Butyl-3-methylimidazolium tetrafluoroborate
- [Bmim] [tca]:
-
1-Butyl-3-methylimidazolium thiocyanate
- [Bmim] [TfO]:
-
1-Butyl-3-methylimidazolium trifluoromethanesulfonate
- [Mbpy] [bti]:
-
1-Butyl-4-methylpyridinium bis[(trifluoromethyl)sulfonyl]imide
- [Mbpy] [BF4]:
-
1-Butyl-4-methylpyridinium tetrafluoroborate
- [Emim] [bti]:
-
1-Ethyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [Emim] [dca]:
-
1-Ethyl-3-methylimidazolium dicyanamide
- [Emim] [ESO4]:
-
1-Ethyl-3-methylimidazolium ethylsulfate
- [Emim] [BF4]:
-
1-Ethyl-3-methylimidazolium tetrafluoroborate
- [Emim] [TfO]:
-
1-Ethyl-3-methylimidazolium trifluoromethanesulfonate
- [N-epy] [bti]:
-
1-Ethylpyridinium bis[(trifluoromethyl)sulfonyl]imide
- [Hpmim] [bti]:
-
1-Heptyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [Hmim] [bti]:
-
1-Hexyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [Hmim] [Cl]:
-
1-Hexyl-3-methylimidazolium chloride
- [Hmim] [PF6]:
-
1-Hexyl-3-methylimidazolium hexafluorophosphate
- [Hmim] [BF4]:
-
1-Hexyl-3-methylimidazolium tetrafluoroborate
- [Hpy] [bti]:
-
1-Hexylpyridinium bis[(trifluoromethyl)sulfonyl]imide
- [Omim] [bti]:
-
1-Octyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [Omim] [PF6]:
-
1-Octyl-3-methylimidazolium hexafluorophosphate
- [Omim] [BF4]:
-
1-Octyl-3-methylimidazolium tetrafluoroborate
- [Pmim] [bti]:
-
1-Pentyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [Prmim] [bti]:
-
1-Propyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide
- [Prmim] [PF6]:
-
1-Propyl-3-methylimidazolium hexafluorophosphate
- [Prmim] [BF4]:
-
1-Propyl-3-methylimidazolium tetrafluoroborate
- [2-HDEA][HCO2]:
-
2-Hydroxydiethylammonium formate
- [Mbupy] [BF4]:
-
2-Methyl-N-butylpyridinium tetrafluoroborate
- [Prmpy] [bti]:
-
3-Methyl-1-propylpyridinium bis[(trifluoromethyl)sulfonyl]imide
- [N1136] [bti]:
-
Dimethylhexyl(i-propyl)ammonium bis[(trifluoromethyl)sulfonyl]imide
- [N1134] [bti]:
-
Dimethylpropylbutylammonium bis[(trifluoromethyl)sulfonyl]imide
- [Mbpyr] [dca]:
-
n-Methyl-n-butylpyrrolidinium dicyanamide
- [P2444] [DEP]:
-
Tributyl(ethyl)phosphonium diethylphosphate
- [N6222] [bti]:
-
Triethylhexylammonium bis[(trifluoromethyl)sulfonyl]imide
- [6,6,6,14-P] [bti]:
-
Trihexyltetradecylphosphonium bis[(trifluoromethyl)sulfonyl]imide
- [6,6,6,14-P] [Cl]:
-
Trihexyltetradecylphosphonium chloride
- [N1114] [bti]:
-
Trimethylbutylammonium bis[(trifluoromethyl)sulfonyl]imide
- [N111,10] [bti]:
-
Trimethyldecylammonium bis[(trifluoromethyl)sulfonyl]imide
- [N1116] [bti]:
-
Trimethylhexylammonium bis[(trifluoromethyl)sulfonyl]imide
- [N8111] [bti]:
-
Trimethyloctylammonium bis[(trifluoromethyl)sulfonyl]imide
- a :
-
Polynomial coefficient in Eq. (1) and adjustable parameter in Eq. (7)
- b :
-
Adjustable parameter in Eq. (7)
- F (0) F (1) :
-
Functions in Eq. (6)
- k :
-
Boltzmann constant
- l :
-
Number of layers
- M :
-
Number of observations
- MW:
-
Molecular weight (g mol−1)
- n :
-
Number of data points
- N :
-
Intermediate layer
- P :
-
Pressure (bar)
- P ch :
-
Parachor ((mN m−1)1/4 cm3 mol−1)
- T :
-
Temperature (K)
- x :
-
Input parameters in Eq. (1)
- y 0 :
-
Output
- Z :
-
Compressibility factor
- γ :
-
Surface tension (mN m−1)
- σ :
-
Hard-sphere segment diameter (0A)
- ε/k :
-
Well depth parameter (K)
- ρ :
-
Density (g cm−3)
- ω :
-
Acentric factor
- b:
-
Boiling
- br:
-
Reduced boiling
- c:
-
Critical
- cal:
-
Calculated
- exp:
-
Experimental
- m:
-
Melting
- r:
-
Reduced
- exp:
-
Experimental
- GMDH:
-
Group method of data handling
- * :
-
Dimensionless
References
Plechkova NV, Seddon KR (2008) Applications of ionic liquids in the chemical industry. Chem Soc Rev 37:123–150
Freire MG, Carvalho PJ, Fernandes AM, Marrucho IM, Queimada AJ, Coutinho JAP (2007) Surface tensions of imidazolium based ionic liquids: anion, cation, temperature and water effect. J Colloid Interface Sci 314:621–630
Atashrouz S, Zarghampour M, Abdolrahimi S, Pazuki G, Nasernejad B (2014) Estimation of the viscosity of ionic liquids containing binary mixtures based on the Eyring’s theory and a modified Gibbs energy model. J Chem Eng Data 59:3691–3704
Kuzmenko I, Rapaport H, Kjaer K, Als-Nielsen J, Weissbuch I, Lahav M, Leiserowitz L (2001) Design and characterization of crystalline thin film architectures at the air-liquid interface: simplicity to complexity. Chem Rev 101:1659–1696
Wang JY, Jiang H, Liu Y, Hu Y (2011) Density and surface tension of pure 1-ethyl-3-methylimidazolium L-lactate ionic liquid and its binary mixtures with water. J Chem Thermodyn 43:800–804
Klomfar J, Souchova M, Patek J (2010) Surface tension measurements with validated accuracy for four 1-alkyl-3-methylimidazolium based ionic liquids. J Chem Thermodyn 42:323–329
Rilo E, Pico J, Garcia-Garabal S, Varela LM, Cabeza O (2009) Density and surface tension in binary mixtures of CnMIM-BF4 ionic liquids with water and ethanol. Fluid Phase Equilib 285:83–89
Atashrouz S, Mirshekar H (2014) Phase equilibrium modeling for binary systems containing CO2 using artificial neural networks. Bulg Chem Commun 46:104–116
Golmohammadi H, Dashtbozorgi Z, Acree WE Jr (2013) Prediction of heat capacities of hydration of various organic compounds using partial least squares and artificial neural network. J Solut Chem 42:338–357
Gardas RL, Coutinho JAP (2008) Applying a QSPR correlation to the prediction of surface tensions of ionic liquids. Fluid Phase Equilib 265:57–65
Lashkarbolooki M, Zeinolabedini Hezave A, Ayatollahi S (2012) Artificial neural network as an applicable tool to predict the binary heat capacity of mixtures containing ionic liquids. Fluid Phase Equilib 324:102–107
Roosta A, Setoodeh P, Jahanmiri A (2012) Artificial neural network modeling of surface tension for pure organic compounds. Ind Eng Chem Res 51:561–566
Zahr Reyhani S, Ghanadzadeh H, Puigjaner L, Recances F (2009) Estimation of liquid-liquid equilibrium for a quaternary system using the GMDH algorithm. Ind Eng Chem Res 48:2129–2134
Atashrouz S, Pazuki G, Alimoradi Y (2014) Estimation of the viscosity of nine nanofluids using a hybrid GMDH-type neural network system. Fluid Phase Equilib 372:43–48
Ketabchi S, Ghanadzadeh H, Ghanadzadeh A, Fallahi S, Ganji M (2010) Estimation of VLE of binary systems (tert-butanol + 2-ethyl-1-hexanol) and (n-butanol + 2-ethyl-1-hexanol) using GMDH-type neural network. J Chem Thermodyn 42:1352–1355
Ghanadzadeh H, Ganji M, Fallahi S (2012) Mathematical model of liquid–liquid equilibrium for a ternary system using the GMDH-type neural network and genetic algorithm. Appl Math Model 36:4096–4105
Pazuki GR, Seyfi Kakhki S (2013) A hybrid GMDH neural network to investigate partition coefficients of Penicillin G Acylase in polymer–salt aqueous two-phase systems. J Mol Liq 188:131–135
Kolbeck C, Lehmann J, Lovelock KRJ, Cremer T, Paape N, Wasserscheid P, Froba AP, Maier F, Steinruck HP (2010) Density and surface tension of ionic liquids. J Phys Chem B 114:17025–17036
Almeida HFD, Freire MG, Fernandes AM, Lopes da Silva JA, Morgado P, Shimizu K, Filipe EJM, Canongia Lopes JN, Santos LMNBF, Coutinho JAP (2014) Cation alkyl side chain length and symmetry effects on the surface tension of ionic liquids. Langmuir 30:6408–6418
Carvalho PJ, Freire MG, Marrucho IM, Queimada AJ, Coutinho JA (2008) Surface tensions for the 1-alkyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide ionic liquids. J Chem Eng Data 53:1346–1350
Tariq M, Serro AP, Mata JL, Saramago B, Esperanca JMSS, Canongia Lopes JN, Rebelo LPN (2010) High-temperature surface tension and density measurements of 1-alkyl-3-methylimidazolium bistriflamide ionic liquids. Fluid Phase Equilib 294:131–138
Kilaru P, Baker GA, Scovazzo P (2007) Density and surface tension measurements of imidazolium-, quaternary phosphonium-, and ammonium-based room-temperature ionic liquids: data and correlations. J Chem Eng Data 52:2306–2314
Sánchez LG, Espel JR, Onink F, Meindersma GW, Haan ABD (2009) Density, viscosity, and surface tension of synthesis grade imidazolium, pyridinium, and pyrrolidinium based room temperature ionic liquids. J Chem Eng Data 54:2803–2812
Pereiro AB, Verdía P, Tojo E, Rodríguez A (2007) Physical properties of 1-butyl-3-methylimidazolium methyl sulfate as a function of temperature. J Chem Eng Data 52:377–380
Wandschneider A, Lehmann JK, Heintz A (2008) Surface tension and density of pure ionic liquids and some binary mixtures with 1-propanol and 1-butanol. J Chem Eng Data 53:596–599
Klomfar J, Součková M, Pátek J (2009) Surface tension measurements for four 1-alkyl-3-methylimidazolium-based ionic liquids with hexafluorophosphate anion. J Chem Eng Data 54:1389–1394
Ghatee MH, Zolghadr AR (2008) Surface tension measurements of imidazolium-based ionic liquids at liquid–vapor equilibrium. Fluid Phase Equilib 263:168–175
Součková M, Klomfar J, Pátek J (2011) Surface tension of 1-alkyl-3-methylimidazolium based ionic liquids with trifluoromethanesulfonate and tetrafluoroborate anion. Fluid Phase Equilib 303:184–190
Muhammad A, Abdul Mutalib MI, Wilfred CD, Murugesan T, Shafeeq A (2008) Thermophysical properties of 1-hexyl-3-methyl imidazolium based ionic liquids with tetrafluoroborate, hexafluorophosphate and bis (trifluoromethylsulfonyl) imide anions. J Chem Thermodyn 40:1433–1438
Klomfar J, Součková M, Pátek J (2011) Temperature dependence of the surface tension and density at 0.1 MPa for 1-ethyl-and 1-butyl-3-methylimidazolium dicyanamide. J Chem Eng Data 56:3454–3462
Tariq M, Freire MG, Saramago B, Coutinho JAP, Lopes JNC, Rebelo LPN (2012) Surface tension of ionic liquids and ionic liquid solutions. Chem Soc Rev 41:829–868
Ghatee MH, Bahrami M, Khanjari N, Firouzabadi H, Ahmadi Y (2012) A functionalized high-surface-energy ammonium-based ionic liquid: experimental measurement of viscosity, density, and surface tension of (2-hydroxyethyl) ammonium formate. J Chem Eng Data 57:2095–2101
Carvalho PJ, Neves CM, Coutinho JA (2010) Surface tensions of bis (trifluoromethylsulfonyl) imide anion-based ionic liquids. J Chem Eng Data 55:3807–3812
Liu QS, Yang M, Yan PF, Liu XM, Tan ZC, Welz-Biermann U (2010) Density and surface tension of ionic liquids [C n py][NTf2](n = 2, 4, 5). J Chem Eng Data 55:4928–4930
Liu QS, Yang M, Li PP, Sun SS, Welz-Biermann U, Tan ZC, Zhang QG (2011) Physicochemical properties of ionic liquids [C3py][NTf2] and [C6py][NTf2]. J Chem Eng Data 56:4094–4101
Xu WG, Li L, Ma XX, Wei J, Duan WB, Guan W, Yang JZ (2012) Density, surface tension, and refractive index of ionic liquids homologue of 1-alkyl-3-methylimidazolium tetrafluoroborate [Cnmim][BF4](n = 2, 3, 4, 5, 6). Chem Eng Data 57:2177–2184
Bandres I, Pera G, Martin S, Castro M, Lafuente C (2009) Thermophysical study of 1-butyl-2-methylpyridinium tetrafluoroborate ionic liquid. J Phys Chem B 113:11936–11942
Restolho J, Serro AP, Mata JL, Saramago B (2009) Viscosity and surface tension of 1-ethanol-3-methylimidazolium tetrafluoroborate and 1-methyl-3-octylimidazolium tetrafluoroborate over a wide temperature range. J Chem Eng Data 54:950–955
Shamsipur M, Beigi AAM, Teymouri M, Pourmortazavi SM, Irandoust M (2010) Physical and electrochemical properties of ionic liquids 1-ethyl-3-methylimidazolium tetrafluoroborate, 1-butyl-3-methylimidazolium trifluoromethanesulfonate and 1-butyl-1-methylpyrrolidinium bis (trifluoromethylsulfonyl) imide. J Mol Liq 157:43–50
Pereiro AB, Santamarta F, Tojo E, Rodríguez A, Tojo J (2006) Temperature dependence of physical properties of ionic liquid 1, 3-dimethylimidazolium methyl sulfate. J Chem Eng Data 51:952–954
Pavel Kordík (2009) Hybrid self-organizing modeling systems, vol. 211. Springer, Berlin Heidelberg
Miran Beigi AA, Abdouss M, Yousefi M, Pourmortazavi SM, Vahid A (2013) Investigation on physical and electrochemical properties of three imidazolium based ionic liquids (1-hexyl-3-methylimidazolium tetrafluoroborate, 1-ethyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide and 1-butyl-3-methylimidazolium methylsulfate). J Mol Liq 177:361–368
Valderrama JO, Rojas RE (2009) Critical properties of ionic liquids. Revisited. Ind Eng Chem Res 48:6890–6900
Kabo GJ, Blokhin AV, Paulechka YU, Kabo AG, Shymanovich MP, Magee JW (2004) Thermodynamic properties of 1-butyl-3-methylimidazolium hexafluorophosphate in the condensed state. J Chem Eng Data 49:453–461
Fernández A, García J, Torrecilla JS, Oliet M, Rodríguez F (2008) Volumetric, transport and surface properties of [bmim][MeSO4] and [emim][EtSO4] ionic liquids as a function of temperature. J Chem Eng Data 53:1518–1522
Gómez E, González B, Domínguez Á, Tojo E, Tojo J (2006) Dynamic viscosities of a series of 1-alkyl-3-methylimidazolium chloride ionic liquids and their binary mixtures with water at several temperatures. J Chem Eng Data 51:696–701
Gardas RL, Freire MG, Carvalho PJ, Marrucho IM, Fonseca IM, Ferreira AG, Coutinho JA (2007) High-pressure densities and derived thermodynamic properties of imidazolium-based ionic liquids. J Chem Eng Data 52:80–88
Machida H, Taguchi R, Sato Y, Smith RL Jr (2010) Measurement and correlation of high pressure densities of ionic liquids, 1-ethyl-3-methylimidazolium l-lactate ([emim][lactate]), 2-hydroxyethyl-trimethylammonium l-lactate ([(C2H4OH)(CH3) 3N][Lactate]), and 1-butyl-3-methylimidazolium chloride ([bmim][Cl]). J Chem Eng Data 56:923–928
Gardas RL, Freire MG, Carvalho PJ, Marrucho IM, Fonseca IMA, Ferreira AGM, Coutinho JAP (2007) P ρ T Measurements of imidazolium-based ionic liquids. J Chem Eng Data 52:1881–1888
Ghatee MH, Zare M, Moosavi F, Zolghadr AR (2010) Temperature-dependent density and viscosity of the ionic liquids 1-alkyl-3-methylimidazolium iodides: experiment and molecular dynamics simulation. J Chem Eng Data 55:3084–3088
Mousazadeh MH, Faramarzi E (2011) Corresponding states theory for the prediction of surface tension of ionic liquids. Ionics 17:217–222
Bogel-Łukasik R, Matkowska D, Zakrzewska ME, Bogel-Łukasik E, Hofman T (2010) The phase envelopes of alternative solvents (ionic liquid, CO2) and building blocks of biomass origin (lactic acid, propionic acid). Fluid Phase Equilib 295:177–185
Pereiro AB, Veiga HI, Esperança JM, Rodríguez A (2009) Effect of temperature on the physical properties of two ionic liquids. J Chem Thermodyn 41:1419–1423
Domanska U, Rekawek A, Marciniak A (2008) Solubility of 1-alkyl-3-ethylimidazolium-based ionic liquids in water and 1-octanol. J Chem Eng Data 53:1126–1132
Sun J, Forsyth M, MacFarlane DR (1998) Room-temperature molten salts based on the quaternary ammonium ion. J Phys Chem B 102:8858–8864
Deetlefs M, Seddon KR, Shara M (2006) Predicting physical properties of ionic liquids. Phys Chem Chem Phys 8:642–649
Rahmati-Rostami M, Behzadi B, Ghotbi C (2011) Thermodynamic modeling of hydrogen sulfide solubility in ionic liquids using modified SAFT-VR and PC-SAFT equations of state. Fluid Phase Equilib 309:179–189
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atashrouz, S., Amini, E. & Pazuki, G. Modeling of surface tension for ionic liquids using group method of data handling. Ionics 21, 1595–1603 (2015). https://doi.org/10.1007/s11581-014-1347-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1347-1