Abstract
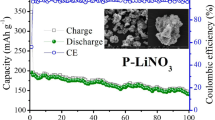
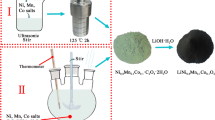
Li1.2Ni0.13Co0.13Mn0.54O2 powders have been prepared through co-precipitation of metal oxalate precursor and subsequent solid state reaction with lithium carbonate. X-ray diffraction pattern shows that the massive rock-like structure has a good layered structure and solid solution characteristic. Scanning electron microscope and transition electron microscope images reveal that the Li1.2Ni0.13Co0.13Mn0.54O2 composed of nanoparticles have the size of 1–2 μm. As a lithium ion battery positive electrode, the Li1.2Ni0.13Co0.13Mn0.54O2 has an initial discharge capacity of 285.2 mAh g−1 at 0.1 C within 2.0–4.8 V. When the cutoff voltage is decreased to 4.6 V, the cycling stability of product can be greatly improved, and a discharge capacity of 178.5 mAh g−1 could be retained at 0.5 C after 100 cycles. At a high charge–discharge rate of 5 C (1,000 mAh g−1), a stable discharge capacity of 121.4 mAh g−1 also can be reached. As the experimental results, the Li1.2Ni0.13Co0.13Mn0.54O2 prepared from oxalate precursor route is suitable as lithium ion battery positive electrode.









Similar content being viewed by others
References
Ellis BL, Lee KT, Nazar LF (2010) Positive electrode materials for Li-ion and Li-batteries. Chem Mater 22:691–714
Goodenough JB, Kim YS (2010) Challenges for rechargeable Li batteries. Chem Mater 22:587–603
He P, Yu HJ, Zhou HS (2012) Layered lithium transition metal oxide cathode towards high energy lithium-ion batteries. J Mater Chem 22:3680–3695
Yu HJ, Zhou HS (2013) High-energy cathode materials (Li2MnO3-LiMO2) lithium-ion batteries. J Phys Chem Lett 4:1268–1280
Thackeray MM, Johnson CS, Vaughey JT, Li N, Hackney SA (2005) Advances in manganese-oxide ‘composite’ electrodes for lithium ion batteries. J Mater Chem 15:2257–2267
Thackeray MM, Kang SH, Johnson CS, Vaughey JT, Benedek R, Hackney SA (2007) Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium ion batteries. J Mater Chem 17:3112–3125
Liu YJ, Liu SB (2013) Effect of cooling method on the electrochemical performance of 0.5Li2MnO3·0.5LiNi0.5Mn0.5O2 cathodes. Ionics 19:477–481
Shi YF, Liu H, Liu GB, You XW (2013) The preparation and electrochemical properties of the Li-excess cathode material Li1+x(Mn0.7Fe0.3)1-xO2 by coprecipitation method. Ionics DOI: 10.1007/s11581-013-0900-7
Lin J, Mu DB, Yin J, Wu BR, Ma YF, Wu F (2013) Li-rich layered composite Li[Li0.2Ni0.2Mn0.6]O2 synthesized by a novel approach as cathode material for lithium ion battery. J Power Sources 230:76–80
Xiang XD, Li XQ, Li WS (2013) Preparation and characterization of size-uniform Li[Li0.131Ni0.304Mn0.565]O2 particles as cathode materials for high energy lithium ion battery. J Power Sources 230:89–95
Yu C, Guan XF, Li GS, Zheng J, Li LP (2012) A novel approach to composite electrode 0.5Li2MnO3·0.5LiNi0.5Mn0.5O2 in lithium-ion batteries with an anomalous capacity and cycling stability at 45.4 °C. Scr Mater 66:300–303
Guo XJ, Li YX, Zheng M, Zheng JM, Li J, Gong ZL, Yang Y (2008) Structural and electrochemical characterization of xLi[Li1/3Mn2/3]O2·(1-x)Li[Ni1/3Co1/3Mn1/3]O2 (0 ≤ x ≤ 0.9) as cathode materials for lithium ion batteries. J Power Sources 184:414–419
Johnson CS, Li NC, Lefief C, Vaughey JT, Thackeray MM (2008) Synthesis, characterization and electrochemistry of lithium batteries electrodes xLiMnO3·(1-x)LiNi0.333Co0.333Mn0.333O2 (0 ≤ x ≤ 0.7). Chem Mater 20:6095–6106
Zhao CH, Kang WP, Liu R, Shen Q (2013) Influence of cobalt on the electrochemical properties of sheet-like 0.5Li2MnO3·0.5LiNi1/3+xCo1/3-2xMn1/3+xO2 as lithium ion battery cathodes. RSC Adv 2:2362–2368
Liu FL, Zhang S, Deng C, Wu Q, Zhang M, Meng FL, Gao H, Sun YH (2012) Cobalt content optimization of layered 0.6Li[Li1/3Mn2/3]O2–0.4LiNi0.5–xMn0.5–xCo2xO2 (0 ≤ x ≤ 0.5) cathode materials prepared by the carbonate coprecipitation. J Electrochem Soc 159:A1591–A1597
Zheng JM, Wu XB, Yang Y (2011) A comparison of preparation method on the electrochemical performances of cathode material Li[Li0.2Ni0.13Co0.13Mn0.54]O2 for lithium ion battery. Electrochim Acta 56:3071–3078
Liu JL, Chen L, Hou MY, Wang F, Che RC, Xia YY (2012) General synthesis of xLi2MnO3·(1-x)LiNi1/3Co1/3Mn1/3O2 nanomaterials by a molten salt methods: towards a high capacity and high power cathode for rechargeable lithium batteries. J Mater Chem 22:25380–25387
Chen Y, Xu GF, Li JL, Zhang YK, Chen Z, Kang FY (2013) High capacity 0.5 Li2MnO3 · 0.5LiNi1/3Co1/3Mn1/3O2 cathode material via a fast co-precipitation method. Electrochim Acta 87:686–692
Zhao TL, Chen S, Li L, Zhang XF, Chen RJ, Belharouak I, Wu F, Amine K (2013) Synthesis, characterization, and electrochemistry of cathode material Li[Li0.2Ni0.13Co0.13Mn0.54]O2 using organic chelating agents for lithium-ion batteries. J Power Sources 228:206–213
Wang ZY, Liu EZ, He CN, Shi CS, Li JJ, Zhao NQ (2013) Effect of amorphous FePO4 coating on the structure and electrochemical performance of Li1.2Ni0.13Co0.13Mn0.54O2 as cathode material for Li-ion batteries. J Power Sources 236:25–32
Jiang KC, Wu XL, Yin YX, Lee JS, Kim J, Guo YG (2012) Superior hybrid cathode material containing lithium-excess layered material and graphene for lithium-ion batteries. ACS Appl Mater Interfaces 4:4858–4863
Wang J, Qiu B, Cao HL, Xia YG, Liu ZP (2012) Electrochemical properties of 0.6Li[Li1/3Mn2/3]O2–0.4LiNixMnyCo1-x-yO2 cathode materials for lithium-ion batteries. J Power Sources 218:128–133
Zhao C, Kang W, Xue Q, Shen Q (2012) Polymerization-pyrolysis-assisted nanofabrication of solid solution Li1.2Ni0.13Co0.13Mn0.54O2 for lithium-ion battery cathodes. J Nanopart Res 14:1240
Tang ZH, Wang ZX, Li XH, Peng WJ (2012) Influence of lithium content on the electrochemical performance of Li1+x(Mn0.533Ni0.233Co0.233)1-xO2 cathode materials. J Power Sources 208:237–241
Lim JH, Bang HJ, Lee KS, Amine K, Sun YK (2009) Electrochemical characterization of Li2MnO3-Li[Ni1/3Co1/3Mn1/3]O2-LiNiO2 cathode synthesized via co-precipitation for lithium secondary batteries. J Power Sources 189:571–575
Hashem AM, EI-Taweel RS, Abuzeid HM, Abdel-Ghany AE, Eid AE, Groult H, Mauger A, Julien CM (2012) Structural and electrochemical properties of LiNi1/3Co1/3Mn1/3O2 material prepared by a two-step synthesis via oxalate precursor. Ionics 18:1–9
Cho TH, Shiosaki Y, Noguchi H (2006) Preparation and characterization of layered LiNi1/3Co1/3Mn1/3O2 as a cathode material by an oxalate co-precipitation route. J Power Sources 159:1322–1327
Lu HQ, Wu F, Su YF, Li N, Chen S, Bao LY (2010) Electrochemical performance of LiNi0.5Mn0.5O2 as cathode material for lithium-ion battery prepared by oxalate co-precipitation route. Acta Phys Chim Sin 26:51–56
Han XY, Meng QF, Sun TL, Sun JT (2010) Preparation and electrochemical characterization of single-crystalline spherical LiNi1/3Co1/3Mn1/3O2 powders cathode material for Li-ion batteries. J Power Sources 195:3047–3052
Kim HJ, Jung HG, Scrosati B, Sun YK (2012) Synthesis of Li[Li0.19Ni0.16Co0.08Mn0.57]O2 cathode materials with a high volumetric capacity for Li-ion batteries. J Power Sources 203:115–120
Wen JW, Zhang DW, Teng YC, Chen CH, Xiong Y (2010) One-step synthesis and improved electrochemical performance of Li(Ni1/3Co1/3Mn1/3)O2 by a modified radiated polymer gel method. Electrochim Acta 55:2306–2310
Li JF, Xiong SL, Li XW, Qian YT (2012) Spinel Mn1.5Co1.5O4 core-shell microspheres as Li-ion battery anode materials with a long cycle life and high capacity. J Mater Chem 22:23254–23259
Acknowledgment
The authors thank the financial supports from Shandong Province (ZR2012BM001), from the National Basic Research Program of China (2011CB935900) and from the NCET Program in the University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, C., Wang, X., Liu, R. et al. Oxalate precursor preparation of Li1.2Ni0.13Co0.13Mn0.54O2 for lithium ion battery positive electrode. Ionics 20, 645–652 (2014). https://doi.org/10.1007/s11581-013-1028-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-013-1028-5