Abstract
The anode supported cell for solid oxide fuel cell, as a modification of the traditional Ni-YSZ anode supported on electrolyte, is examined in this work. The materials obtained on the base of citric method are presented and investigated in this work. The materials consisted of 40 wt.% Ni/YSZ, 50 wt.% Ni/YSZ and 60 wt.% Ni/YSZ were obtained. The base Ni/YSZ materials are tested on the two ways: (a) aging tests and (b) sintering tests. All the materials after aging and sintering are tested by the impedance spectroscopy. The results of electrical conductivity for samples before and after aging show that only in the case of 40 wt.% Ni/YSZ, sample loses of metallic conductivity after 500 h of heating. The other samples reveal metallic conductivity even after long period of aging. The tests of sintering temperature show that this process does not affect significantly on electrical conductivity of the materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anode supported cell for solid oxide fuel cell (SOFC) has been extensively studied for about 10 years [1–3]. Such cell uses Ni/YSZ (nickel–yttria-stabilized zirconia) cermet as an anode material. The main advantages of the Ni/YSZ cermet are high mixed electronic–ionic conductivity, good catalytic activity with respect to hydrogen oxidation, high mechanical strength and relatively low price. What is more, a very important for long-life of anode is its thermal stability. Traditionally, this material is prepared by mechanical mixing of appropriate amounts of nickel or NiO with YSZ. Nowadays, the methods of wet chemical synthesis are often used in order to obtain homogenous and nanosize particles. Some original methods of synthesis described in literature are co-precipitation by NaOH method [4], nitrates combustion [5], combustion citrate–nitrate method [6], classical Pechini method [7], combustion of nitrate–glycine gel [8], sol–gel method with sucrose and pectin addition [9], complex-gel conversion process by EDTA [10], self-assembled NiO-YSZ directionally solidified eutectics method [11] and precipitation of NiO or Ni precursors on YSZ [12, 13]. The characteristic feature of these methods is that synthesized materials have uniform nanostructure and high level porosity without a necessity of using a pore former.
The research carried out up to now proves that microstructure of materials strongly depends on particular preparation method. What is more important, microstructure of cermets affects mechanical and electrical properties of material [4, 14, 15]. The fundamental requirement for microstructure of anode materials is the continuity of phases and appropriate length of triple phase boundary (TPB). The first one ensures metallic as well as ionic conductivity, while the second is responsible for catalytic activity with respect to anode electrochemical reactions. As an example of a co-precipitation method, Sato et al. [4] showed that changes in synthesis conditions have strong influence on anode’s performance. Pratihar et al. [14] showed that electrical conductivity of Ni/YSZ cermet materials increases with decreasing level of samples porosity and investigated how an addition of pore former can influence material’s conductivity. Some authors reported microstructure evolution Ni/YSZ cermet materials under cell operation temperature. Marinsek and Zupan [6] showed that during the reduction of NiO to metallic nickel, mean particle size is growing but sintering temperature above 1,200 °C did not significantly influence the density of the material. Kim et al. [7] tested conductivity of the material (41 % porosity and 50 vol% of Ni/8YSZ) after heat treatment in hydrogen at 1,000 °C for 100 h. They observed that there was no change in the conductivity after heating. They also reported a change in the thermal expansion coefficient (TEC) in the temperature range 300–1,000 °C for samples with different porosity level (23, 34 and 41 %). All samples showed similar values of TEC at 1,000 °C, which indicates that TEC was independent of the porosity. Radovic et al. [16] obtained similar results. Up to now, the thermal durability was mostly examined for classically prepared anodes (with NiO and YSZ powders) [7, 17–21]. Only a few papers concern with a durability of materials obtained in other ways [6, 7, 11, 22]. Hattori et al. [22] investigated electrical conductivity of pure YSZ (8.0–10.0 mol% Y2O3) with annealing at 1,000 °C. They showed that only materials 9.5YSZ and 10YSZ reveal no conductivity decrease after annealing (even for 1,000 h). In the case of 8.0YSZ and 8.5YSZ materials, conductivity significantly decreased with time although initial conductivities for these samples were the highest from the all. The others consider influence of annealing on Ni/YSZ cermet. Kim et al. [7] examined a material obtained by precipitation of nickel by Pechini method on the YSZ surface. Their thermal/redox tests consisted in oxidation (under air atmosphere) and reduction (under hydrogen atmosphere) of samples at 800 °C. The authors claim that their composite material has excellent tolerance against thermal and redox cycling in comparison to mechanically mixed NiO and YSZ powders. They presented microstructure changes (on the base of SEM images) of Ni/YSZ materials which confirmed nickel coarsening in the case of material made from NiO/YSZ mixing powder and much smaller coarsening effect for NiO/YSZ composite material. The interesting results obtained Laguana-Bercero et al. [11] for materials with channelled microstructure. They examined Ni/YSZ sample after 300 h of aging in the H2/N2 atmosphere at 900 °C. The authors found that the same samples before and after aging showed no signs of degradation in respect of porosity and electronic conductivity of material. The channelled Ni/YSZ cermet is potentially an excellent candidate for anode material in SOFC, but the method of its synthesis is complicated in comparison to the other methods. In this paper, we studied the effect of the sintering temperature and aging processes on structural and electrical properties of Ni/8YSZ cermet SOFC anode materials obtained by citric method.
Experimental
Preparation of materials
Synthesis of initial material
Solutions of nickel, yttrium and zirconyl nitrates were prepared and mixed in proper ratio in order to obtain Ni/8YSZ. The cermet materials containing 40, 50 and 60 wt.% Ni in the mixture with 8YSZ (8 mol% yttria–92 mol% zirconia) were manufactured in this way. An appropriate amount of citric acid monohydrate (the molar ratio of fuel to nitrate was 1:1 with c.a. of 5 % excess of acid) was added to metal nitrates solution. The mixed solution was put in open glass beaker and stirred for a few hours on a hot plate (around 220 °C). Afterwards, the solution turned into grey-green gel. This gel was heated on the burner in air. Then the disk pallets were pressed and sintered at 800 °C in 10 % H2/90 % Ar mixture for 3 h.
After characterisation of structural, microstructural and electrical properties of the initial materials, they were subjected additionally to aging and sintering tests. The aging tests consist of heating the cermet pallets at 800 °C in the flow of 10 % H2/90 % Ar mixture for 200 h. After this time, the pallets were sampled for SEM and electric measurements. The rest of the pallets were left in heater up to 500 h, and after this time, they were also tested in the same way. The sintering tests relied on sintering of samples in the flow of synthetic air during 6 h at various temperatures within the range 800–1,300 °C. Next, the samples were reduced at 800 °C in the mixture 10 % H2 + 90 % Ar for 2 h. All samples were tested by the impedance spectroscopy after sintering, and total porosity was determined from geometrical density measurements of the pallets.
Characterisation of samples
The phase composition of the powders was determined by XRD analysis using CuKa radiation within 2Θ range 20–90° by means of Philips X’Pert Pro diffractometer. The SEM observations were done using JROL 5400 scanning electron microscope with EDS analyser.
The open porosity was calculated using water saturation method. The total porosity was determined by relative geometrical density measurements assuming that densities of metallic Ni and 3YSZ are equal to 8.908 and 6.06 g/cm3, respectively.
Values of TEC measurements were determined using measuring instrument and “size changing” transducer provided by a DIL 402 C equipment from NETZSCH. Cylindrical samples of diameter 10 mm and thickness of 1 mm were used in the experiments. The measurements were performed in the Ar atmosphere containing 5 % H2; a rate of temperature change was 5 °C/min within the temperature range of 20–800 °C. The values of TEC were calculated using the linear regression approximation.
The electric properties measurements were carried out with Solatron SI 1260 Impedance/Gain-Phase Analyzer with the SI 1296 dielectric interface for temperature range between 20 and 700 °C at the frequencies ranging from 0.1 to 106 Hz. A flowing gas atmosphere of 10 % H2 in Ar was used.
Results and discussions
Characterisation of the initial cermet material
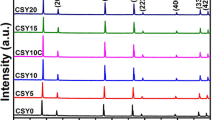
The X-ray analysis showed that for all compositions studied, the Ni/8YSZ powders after calcination consisted only of cubic phase ZrO2 and cubic nickel oxide. After heating in 10 % H2/90 % air mixture, NiO reduces to metallic nickel. The example of X-ray diffraction patterns for 50 % Ni/8YSZ powder after calcination and after reduction is shown in Fig. 1.
The crystallite size of powders after calcination determined from the cubic ZrO2 (111) and cubic NiO (002) peaks broadening was equal to 15.8 ± 0.5 and 38.0 ± 0.5 nm, respectively. The crystallite sizes after reduction of the samples were 22.5 ± 0.5 nm for ZrO2 (111) and 145.0 ± 0.5 nm for cubic Ni (111). A significant difference in crystallite sizes of NiO and Ni confirms that the cermet heating in reducing atmosphere leads to growth of nickel crystallites. This effect is consequently connected with the TPB reduction. Marinsek and Zupan [6] in a paper mentioned above explained an effect of excessive grain growth of nickel particles as a consequence of different surface energies of nickel and YSZ resulting in poor adhesion of metallic nickel to the zirconia.
The results of total and open porosity measurements after reduction of samples (in mixture 10 % H2 in Ar) are shown in Table 1. The values of total porosity calculated from the geometrical density fit in the range of 62–67 %. The open porosity is about 10–13 % lower. All values are suitable for SOFC anode. It is significant that high degree of porosity was reached without the application of any pore formers.
SEM micrographs (Fig. 2) show that the initial samples with various content of nickel contain nano-sized crystallites. Moreover, crystallites derived from two phases, namely nickel and zircon oxide, cannot be distinguished (but they create common aggregates).
The results of dilatometric studies within temperature range of 20–800 °C are presented in Fig. 3. The thermal expansion coefficient was determined in the temperature range 400–700 °C, where linear dependence of length vs. temperature T can be observed. The literature TEC values for pure nickel and fully dense 8YSZ are 17.0 × 10−6 K−1 [23] and 11.0 × 10−6 K−1, respectively [24]. An analogous measurement of TEC was carried out for porous 8YSZ prepared by the same citric method. The value of TEC for this material is equal to 8.7 × 10−6 K−1. TEC values of tested samples are presented in Table 2. These values are similar to each other and fall between the TEC value of fully dense 8YSZ and for porous 8YSZ, which confirms the compatibility between anode and the electrolyte materials.
Materials after aging tests
Microstructures of the cermet samples after 500 h of heating at 800 °C in H2/Ar mixture are presented in Fig. 2b, d, f. One can observed that obtained materials have nanometric sizes before and after aging and that after long sintering the samples are more solid than initial ones. As in the case of images presented by Laguana-Bercero et al. [11] (the same blow-up), it is difficult to find in Fig. 2 any signs of degradation of cermets examined in this paper. SEM images shown by Kim et al. [7] had different enlargement (ten times) than in our studies, but it can be noticed that microstructure of their materials is similar to ours samples.
Figure 4 shows dilatometric measurements for samples after aging process. The values of the TEC for all three samples of Ni/YSZ materials calculated in the temperature range of 400–700 °C are almost the same, which suggests no significant dependence of thermal properties of cermets on Ni content for this temperature range. On the other side, these values are an order of magnitude lower than TEC values estimated for the same samples before aging. This is an interesting effect which needs further studies.
Electrical properties
The effect of aging process on electrical properties of cermet samples was analysed by comparison with respective data measured for initial samples. The impedance spectroscopy measurements showed that all initially prepared cermets 40 % Ni/8YSZ, 50 % Ni/8YSZ and 60 % Ni/8YSZ samples exhibit an increase in electrical resistance with temperature (Fig. 5). It is a characteristic behaviour for metallic conductivity. An analysis of impedance spectra revealed that equivalent circuit presented in Fig. 6a represents well the impedance data of basic materials. The elements R1 and L1 correspond to electrode resistance effect (values of R1 nearly do not change with temperature increasing) and inductance of the wires, respectively, whereas R2-L2 can be attributed to nickel paths which are responsible for metallic conductivity of cermet (values of R2 raise with temperature increasing). Figure 7 illustrate the Nyquist plots for all initial samples.
The electrical properties of materials after aging tests are various, depending on nickel content (Fig. 8). The classical increase of resistance with temperature can be observed only for the 50 % Ni/8YSZ material. It demonstrates that metallic character of conductivity for this material after aging process is retained (Fig. 8b). In contradiction to 50 % Ni/8YSZ material, 40 % Ni/8YSZ sample behaves like dielectric which shows decreasing of resistivity with temperature growth (it corresponds to ionic conductivity ) (Fig. 8a). Metallic conductivity disappears after aging of initial samples as a result of disconnecting of nickel paths. For 60 % Ni/8YSZ sample, the Nyquist plots are more complex (Fig. 8c); it can, however, be noticed that the material demonstrates a metallic conductivity component in the range of low temperatures (100–300 °C) and behaves as semiconductor in the higher temperatures, which was identified on the basis of presence of positive values in the points on Z′ vs. Z″ plot. Untypical character of impedance spectra for 60 % Ni/8YSZ is probably connected with agglomeration of nickel particles in a sample with such large content of Ni, but undoubtedly this effect requires further investigations.
The example of parameters determined for spectra (obtained for 50 % Ni/YSZ) is presented in Table 3. The parameters are calculated on the basis of circuits shown in Fig. 6a (for initial sample and for sample after aging during 200 h) and Fig. 6b (sample after aging during 500 h). It can be observed that aging process carried out for 200 and for 500 h does not have influence on electrical properties of 50 % Ni/YSZ cermet. The values of specific resistance ρ 2 (for initial material and for material after 200 h of aging) and ρ (for material after 500 h of aging) are close to each other. The values of resistivity slightly increase with the time of aging (Fig. 9). This is a confirmation that material 50 % Ni/YSZ obtained by citric method is the most interesting one due to its thermal stability. At the same time, it is important to remember (even in case of this material) that a long time of aging can lead to deterioration of electrical properties of the anode.
Sintering tests
The pellets consisting of initial materials were sintered at various temperatures (800, 1,000, 1,200 and 1,300 °C). The total porosity after sintering was estimated. For all the compositions of cermets sintered at 800 °C, porosity values are lower than those in case of initial samples because nickel, which is a component of cermets, undergoes oxidation during heating in air. The values of porosity decreased with increasing temperature which is related to processes of cermet sintering. The porosity increased after reduction of nickel oxide due to the release of steam. An example of porosity versus sintering temperature dependence of 50 % Ni/8YSZ cermet is shown in Fig. 10. It is very important that even for sample sintered at 1,300 °C, the value of porosity remain close to 50 % since such porosity is suitable for applying this cermet as anode material.
The measurements of electrical properties for all cermets studied, regardless of sintering temperature, revealed metallic conductivity (an example of Nyquist plots for 50 % Ni/YSZ sintered in 1,200 °C is presented in Fig. 11). A comparison of admittance measured for the samples at all sintering temperatures for different nickel content are shown in the Nyquist plots (Fig. 12). All presented curves show semicircles describing electron conductivity (equivalent circuit Fig. 6a). It was observed that metallic conductivity appeared for the all sintering temperatures. Nevertheless, the most important fact is that sintering of materials in the range of 800–1,200 °C does not change the value of metallic conductivity. Only in the case 40 % Ni/YSZ sample sintered at 800 °C, the admittance was twice lower in comparison to other sintering temperatures, and it is likely that an increase of sintering temperature improved contacts between nickel grains in this sample.
Conclusions
The citric method is very useful for obtaining nanosize, homogenous materials. Thermal treatment of Ni/YSZ cermet materials in hydrogen leads to reduction of nickel oxide to metallic nickel with simultaneous growth of nickel particles. Reduction also results in decreasing of material’s density while increasing its porosity level. The values of porosity for materials obtained by citric method (close to 50 %), even for materials sintered in 1,300 °C, make these cermets suitable as anode material. TEC values show that all obtained materials reveal thermal compatibility with pure YSZ and all three compositions of Ni/YSZ cermet, initial materials, reveal metallic conductivity.
Aging of materials for 200 h does not alter their conductivity, but extension of aging time to 500 h leads to disappearance of metallic conductivity in a 40 % Ni/8YSZ sample. It is consistent with breaking of nickel paths in this material. After such a long time at hydrogen atmosphere, 50 % Ni/8YSZ and 60 % Ni/8YSZ materials retain metallic conductivity. In the case of 60 % Ni/8YSZ, the values of conductivity are at least one order of magnitude lower than those of 50 % Ni/8YSZ. This demonstrates that only in 50 % Ni/8YSZ sample, material continuity of nickel paths remained unchanged. In 60 % Ni/8YSZ materials, the nickel probably undergoes agglomeration. The sintering tests show that regardless of sintering temperature (in the range 800–1,200 °C), metallic conductivities are almost constant for the same materials.
References
Kim SD, Moon H, Hyun S-H, Moon J, Kim J, Lee H-W (2007) Ni-YSZ cermet anode fabricated from NiO-YSZ composite powder for high-performance and durability of solid oxide fuel cells. Solid State Ionics 178:1304–1309
Park YM, Lee HJ, Bae HY, Ahn JS, Kim H (2012) Effect of anode thickness on impedance response of anode-supported tubular solid oxide fuel cell. Int J Hydrogen Energy 37:4394–4400
Lanzini A, Leone P (2010) Experimental investigation of direct internal reforming of biogas in solid oxide fuel cells. Int J Hydrogen Energy 35:2463–2476
Sato K, Okamoto G, Naito M, Abe H (2009) NiO/YSZ nanocomposite particles synthesized via co-precipitation method for electrochemically active Ni/YSZ anode. J Power Sources 193:185–188
Priyatham T, Bauri R (2010) Synthesis and characterization of nanocrystalline Ni-YSZ cermet anode for SOFC. Mater Charact 61(1):54–58
Marinsek M, Zupan K (2010) Microstructure evaluation of sintered combustion-derived fine powder NiO-YSZ. Ceram Int 36:1075–1082
Kim SD, Moon H, Hyun S-H, Moon J, Kim J, Lee H-W (2006) Performance and durability of Ni-coated YSZ anodes for intermediate temperature solid oxide fuel cells. Solid State Ionics 177:931–938
Kakade MB, Ramanathan R, Das D (2011) Gel-combustion, characterization and processing of porous Ni-YSZ cermet for anodes of solid oxide fuel cells (SOFCs). Ceram Int 37:195–200
Suciu C, Hoffmann AC, Doroloti E, Tetean R (2008) NiO/YSZ nanoparticles by new sol–gel route. Chem Eng J 140:586–592
Shao G-Q, Cai H, Xie J-R, Duan X-L, Wu B-L, Yuan R-Z, Guo J-K (2003) Preparation of nanocomposite Ni/YSZ cermet powder by EDTA complex-gel conversion process. Mater Lett 57:3287–3290
Laguana-Bercero MA, Larrea A, Merino RI, Pena JI, Orera VM (2005) Stability of channeled Ni-YSZ cermets produced from self-assembled NiO-YSZ directionally solidified eutectics. J Am Ceram Soc 88(11):3215–3217
Han KR, Jeong Y, Lee H, Kim C-S (2007) Fabrication of Ni/YSZ anode material for SOFC via mixed NiO precursors. Mater Lett 61:1242–1245
Drożdż-Cieśla E, Wyrwa J, Pyda W, Rękas M (2012) A new method of preparing Ni/YSZ cermet materials. J Mater Sci 47:2807–2817
Pratihar SK, Dassharma A, Maiti HS (2005) Processing microstructure property correlation of porous Ni-YSZ cermet anode for SOFC application. Mater Ress Bull 40:1936–1944
Clemmer RMC, Corbin SF (2009) The influence of pore and Ni morphology on the electrical conductivity of porous Ni/YSZ composite anodes for use in solid oxide fuel cell applications. Solid State Ionics 180:721–730
Radovic M, Lara-Curzio E, Trejo RM, Wang H, Porter WD (2007) Advances in solid oxide fuel cells II. Wiley, Hoboken, pp 79–85
Primdahl S, Mogensen M (2000) Durability and thermal cycling of Ni/YSZ cermet anodes for solid oxide fuel cell. J Appl Electrochem 30:247–257
Baek S-W, Bae J (2011) Anodic behavior of 8Y2O3-ZrO2/NiO cermet using an anode-supported electrode. Int J Hydrogen Energy 36:689–705
Ettler M, Timmermann H, Malzbender J, Weber A, Menzler NH (2010) Durability of Ni anodes during reoxidation cycles. J Power Sources 195:5452–5467
Talebi T, Sarrafi MH, Haji M, Raissi B, Maghsoudipour A (2010) Investigation on microstructures of NiO-YSZ composite and Ni-YSZ cermet for SOFCs. Int J Hydrogen Energy 35:9440–9447
Laurencin J, Delette G, Morel B, Lefebvre-Joud F, Dupeux M (2009) Solid oxide fuel cells damage mechanisms due to Ni-YSZ re-oxidation: case of the anode supported cell. J Power Sources 192:344–352
Hattori M, Takeda Y, Sakaki Y, Nakanishi A, Ohara S, Mukai K, Lee J-H, Fukui T (2004) Effect of aging on conductivity of yttria stabilized zirconia. J Power Sources 126:23–27
Krikorian OH (1960) UCRL—6132
Männer R, Ivers-Tiffée E, Wersing W (1991) In Grosz F, Zegers P, Singhal SC, Yamamoto O (eds) SOFC II. Commission of the European Communities, Luxemburg, L. EUR-13564-EN. 715
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Drożdż, E., Wyrwa, J. & Rękas, M. Influence of sintering temperature and aging on properties of cermet Ni/8YSZ materials obtained by citric method. Ionics 19, 1733–1743 (2013). https://doi.org/10.1007/s11581-013-0914-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-013-0914-1