Abstract
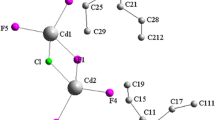
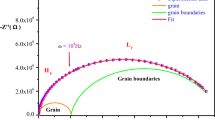
The present paper reports the synthesis, crystal structure, 13C and 111Cd cross-polarization magic-angle spinning nuclear magnetic resonance(CP-MAS-NMR) analysis and ac conductivity for a new organic–inorganic hybrid salt, [C7H12N2][CdCl4]. The compound crystallizes in the triclinic system, space group P\( \overline 1 \), with unit cell dimensions: a = 7.1050(3) Å, b = 8.9579(3) Å, c = 9.4482(3) Å, α = 81.415(1)°, β = 89.710(2)°, γ = 85.765(1)°, V = 592.97(4) Å3, and Z = 2. The asymmetric unit is composed of one-2,4-diammonium toluene cation and one [CdCl4]2− anion. The Cd atom is in a slightly distorted octahedra coordination environment. Its structure can be described by infinite chains of CdCl6 octahedron linked to organic cations by a strong charge-assisted N–H∙∙∙Cl interactions in order to build organic–inorganic layers staked along \( \left[ {0\overline 1 1} \right] \) direction. The solid state 13C CP-MAS-NMR spectra has shown seven isotropic resonances, confirming the existence of seven non-equivalent carbon atoms, which is consistent with crystal structure determined by X-ray diffraction. As for 111Cd MAS-NMR, it has shown one cadmium site with isotropic chemical shift observed at 167.2 ppm. The complex impedance of the compound has been investigated in the temperature range of 403–460 K and in the frequency range of 200 Hz–5 MHz. The impedance plots have shown semicircle arcs at different temperatures and an electrical equivalent circuit has been proposed to explain the impedance results. The circuits consist of the parallel combination of bulk resistance R p and constant phase elements.













Similar content being viewed by others
References
Min KS, Suh MP (2000) J Am Chem Soc 122:6834
Fan J, Gan L, Kawaguchi H, Sun WY, Yu KB, Tang WX (2003) Chem Eur J 9:3965
Fletcher AJ, Cussen EJ, Bradshaw D, Rosseinsky MJ, Thomas KMJ (2004) Am Chem Soc 126:9750
Chun H, Dybtsev DN, Kim H, Kim K (2005) Chem Eur J 11:3521
Mines GA, Tzeng BC, Stevenson KJ, Li J, Hupp JT (2002) Angew Chem Int Ed 41:154
Yoo SK, Ryu JY, Lee JY, Kim C, Kim SJ, Kim Y (2003) Dalton Trans 1454. doi:10.1039/B301082A
Han H, Zhang S, Hou H, Fan Y, Zhu Y (2006) Eur J Inorg Chem 8:1594
Dybtsev DN, Nuzhdin AL, Chun H, Bryliakov KP, Talsi EP, Fedin VP, Kim K (2006) Angew Chem Int Ed 45:916
Hasegawa S, Horike S, Matsuda R, Furukawa S, Mochizuki K, Kinoshita Y, Kitagawa SJ (2007) Am Chem Soc 129:2607
Kato Y, Ichii D, Ohashi K, Kunugita H, Ema K, Tanaka K, Takahashi T, Kondo T (2003) Solid State Commun 128:15
El-Korashy A, Brik MG (2005) Solid State Commun 135:298
Riou-Cavellec M, Albinet C, Greneche JM, Ferey G (2001) J Mater Chem 11:3166
Lach G, Laskowski L, Kityk IV, Kapustianyk V, Rudyk V, Shchur Y, Tkaczyk S, Swiatek J, Piasecki M (2007) J NonCrystalline Solid 353:4353
Hong X, Ishihara T, Nurmikko AV (1992) Phys Rev B45:6961
Fujisawa J, Ishihara T (2004) Phys Rev B70:113203
Pons J, Antń JG, Bardia MF, Calvet T, Ros J (2009) Inorg Chim Acta 362:2698
Salamakha P, Sologub O, Demchenko P, Righi L, Bocelli G (2002) J Alloy Compd 335:142
Olekseyuk ID, Gulay LD, Dydchak IV, Piskach LV, Parasyuk OV, Marchuk OV (2002) J Alloy Compd 340:141
Smart & SAINT Software Reference Manuals, Version 6.22 (2001). Bruker AXS, Analytical Instrumentation, Madison, Wisconsin, USA
Sheldrick GM (2004) SADABS. University of Göttingen, Germany
Macardle P (1996) J Appl Cryst 29:306
Sheldrick GM (1986) SHELXS-86, Program for Crystal Structure solution. University of Göttingen, Germany
Sheldrick GM (1997) SHELXL-97, Program for crystal structure refinement. University of Göttingen, Germany
Massiot D, Theile H, Germany A (1994) Bruker Rep 43:140
Brown ID (1976) Acta Cryst A32:24
Jin ZM, Shun N, Lü YP, Hu ML, Shen L (2005) Acta Cryst C61:m43
Rademeyer M (2005) Acta Cryst E61:m304
Chaabane I, Hlel F, Guidara K (2008) PMC Physics B 1:11. doi:10.1186/1754-0429-1-11
Qiang Y, Qin-jing M, Xiao-zeng Y, Xiao-ying H (1996) Acta Cryst C52:33
Hlel F, Thouvenot R, Smiri L (2005) Phys Stat Sol (b) 242:1243
Galka HC, Gade LH (2004) Inorg Chim Acta 357:1725
Sakida S, Kawamoto Y (2002) J Phys Chem Solid 63:151
Nadeem M, Akhtar MJ, Khan AY (2005) Solid State Commun 134:431
Muralidharan P, Venkateswarlu M, Satyanarayana N (2005) J NonCryst Solid 351:583
Rao KS, Prasad DM, Krishna PM, Tilak B, Varadarajulu KC (2006) Mater Sci Eng B133:141
Ayouchi R, Leien D, Martin F, Gabas M, Dalchiele E, Ramos-Barrodo JR (2003) Thin Solid Films 426:68
Fan CL, Ciardullo D, Huebrier W (2003) Mater Sci Eng B100:1
Réau JM, Rossignol S, Tanguy B, Rojo JM, Herrero P, Rojas RM, Sanz J (1994) Solid State Ionics 74:65
Jonscher AK (1974) Nature 250:191
Jain H, Mundy JN (1987) J NonCryst Solids 91:315
Dyre JC, Schroder TB (2000) Rev Mod Phys 72:873
Ben Rhaiem A, Zouari N, Guidara K, Gargouri M, Daoud A (2005) J Alloys Compd 387:1–5
Hazara S, Chosh A (1996) Philosophical B74(3):235
Mott NF, Davis EA (1979) Electronic Processes in Non-Crystalline Materials, 2nd edn. Clarendon, Oxford
Botthger H, Bryksin VV (1976) Phys Status Solidi B78:415
Long AR (1982) Adv Phys 31:553
Elliott SR (1987) Adv Phys 36:135
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(CIF 19.3 kb)
Rights and permissions
About this article
Cite this article
Jarboui, A., Ousleti, A., Adil, K. et al. A new one-dimensional hybrid material lattice: AC conductivity and structural characterization of [C7H12N2][CdCl4]. Ionics 17, 145–155 (2011). https://doi.org/10.1007/s11581-010-0495-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-010-0495-1