Abstract
1-(2Aminoethyl) pyrrolidinium tetrachlorocobaltat (C6H16N2) CoCl4 has been prepared and analyzed by X-ray diffraction. The compound crystallizes in the orthorhombic system with space group P212121. The unit cell dimensions are a= 7.3279(13), b= 13.051(2) and c= 13.831(2) Å with Z= 4. The crystal structure of this salt was solved by Patterson methods and refined by full-matrix least squares on F 2 to the final values of R=0.0486 and wR =0.1347. The structure consists of diprotonated 1-(2aminoethyl) pyrrolidinium cations and monomeric CoCl\(_{4}^{2-}\) These entities are interconnected by means of hydrogen bonding contacts forming a threedimensional network. Magnetization was used to investigate the magnetic properties. This compound exhibits an antiferromagnetic (AFM) to paramagnetic (PM) phase transition at a temperature (T N) lower than 2 K. The values of paramagnetic Curie–Weiss temperature (𝜃 cw) and the exchange parameter (J/ K B) emphasize the existence of an antiferromagnetic interaction between the neighboring cobaltions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The organic–inorganic hybrid materials based on metal halide units have been the subject of many recent investigations because of their interesting structural and magnetic properties [1–5]. The potential applications of these compounds have drawn the attention of numerous scientists due to their catalysis, bioinorganic, biomimetic and coordination chemistry [6]. They have also played a significant role in the development of the understanding of lowdimensional magnetic systems and, more recently, in semiconducting materials [7]. As known magnetic susceptibility is an important tool for the magnetic characterization of the material. One of the beststudied groups of halide transition-metal complexes, both in solution and the solid state, is the cobalt(II) halide complexes. An extensive study has been performed on magnetic compounds in which the 3-d magnetic ions are tetrahedrally coordinated by the ligand [C 6 H 18 N 3] [8].
An extensive study has been performed on magnetic compounds in which magnetic and thermal behavior of the series of isostructural N-methylmorpholinium compounds (nmmH) 2[MX 4] where M = Mn 2+, Co 2+ or Cu 2+ and X = Cl − or Br − [nmmH =N-methylmorpholinium]. The compounds are all isomorphous and exhibit weak onedimensional antiferromagnetic interaction, with a contribution from singleion anisotropy in the case of the cobalt complexes [9].
In this paper, we report the preparation, crystal structure and magnetic properties of the compound 1-(2aminoethyl) pyrrolidinium tetrachlorocobaltat.
2 Experimental
2.1 Crystal Chemistry
The (C6H16N2) CoCl4 crystals were obtained by dissolving in a concentrated HCl (36 %) solution a stoichiometric mixture of 1-(2aminoethyl) pyrrolidine and a solution of CoCl 2⋅ 6H 2O in a minimum volume of water. The resulting aqueous solution was left to evaporate slowly at room temperature. After several weeks of evaporation, the product separates as crystals, which were isolated by filtration and dried in air. The new compounds show satisfactory elemental analyses.
2.2 Crystal Structure
Intensity data were collected using an Enraf–Nonius Kappa CCD diffractometer with graphite-monochromator (MoK g, λ=0.71073Å). The positional parameters for the heavy atoms were obtained from a three-dimensional Patterson map, while the non-H atoms were found from successive difference Fourier maps. The structure was refined by full-matrix least squares using anisotropic temperature factors for all non-hydrogen atoms and the hydrogen atoms were localized and optimized to restraint positions. Calculations were performed with the programs SHELXS and SHELXL [10], using the scattering factors enclosed therein. The crystal data, collected reflections and parameters of the final refinement are reported in Table 1. The final reliability factor converged to R= 0.0486 and wR = 0.1347.
2.3 Magnetic Measurements
Magnetic measurements were obtained in the temperature range 2–15 K, with an external magnetic field varying from 0.05 to 5 T.
3 Results and Discussion
3.1 Structure Description
The final atomic coordinates with U eq of non-hydrogen atoms are given in Table 2. Interatomic distances, bond angles, and the hydrogen bond scheme are listed in Table 3.
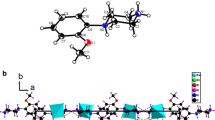
The structure of the title compound consists of isolated monomeric [CoCl 4] 2− and diprotonated 1-(2aminoethyl) pyrrolidinium as shown in Fig. 1.
An examination of the structure shows that the atomic arrangement can be described by an alternation of organic and inorganic species. The anionic groups are built up of isolated tetrahedral of [CoCl 4] 2− arranged in sandwich between two organic species (Fig. 2).
The anion exhibits tetrahedral geometry, with the Co(II) ion surrounded by four chlorine atoms, with Cl–Co–Cl angles ranging from 102.78(1) ∘ to 117.36(8) ∘. The mean Co–Cl bond length, 2.28 Å, is close to those observed in similar complex [11, 12].
Generally, the Co–Cl bond lengths and Cl–Co–Cl bond angles in the [CoCl 4] 2− anion are not equal to one another, but vary with the environment around the Cl atoms. In the title compound, the Co–Cl 1 and Co–Cl 4 are obviously longer than Co–Cl 2 and Co–Cl 3. The Co–Cl 4 bond length 230(18) Å is the longest one and the Co–Cl 2 bond 226(18) Å the shortest. Owing to the obvious differences in the Co–Cl bond lengths and the Cl–Co–Cl angles, the coordination geometry of the Co atom can be regarded as being a distorted tetrahedron.
A projection of the structure in the plane (bc) shows that the organic and inorganic groups alternate, whichever direction is chosen (Fig. 2). In the title compound, the mean plan through the pyrrolidine ring built up by the atoms (N 1, C 1, C 2, C 3, C 4) is less planar (r.m.s. deviation = 0.144(1) A ∘). In fact, the conformation of the ethylamine side chain with respect to pyrrolidine ring is usually described by torsion angles C 1–N 1–C 5–C 6 (τ 1) and N 1–C 5–C 6–N 2 (τ 2). Cation has an extended conformation with τ 1= 164.19(1) and τ 2= 156.51(1). The C–C bond lengths vary from 1.4(11) to 1.5(2) Å. The C–N bond lengths vary from 1.43(11) to 1.50(12) Å. Table 3 reports the main geometrical features of (C 6 H 16 N 2)2+.
In (C6H16N2) CoCl4, the organic species interact with the inorganic chains via N–H ∼ Cl hydrogen bonds, forming a threedimensional network (Fig. 2). All the hydrogen bonded to the nitrogen atoms participate in the formation of (N–H ⋯ Cl) hydrogen bonding with distances 2.297 to 2.406 Å. Details of the hydrogen bonding scheme are reported in Table 3.
3.2 Magnetic Properties
In Fig. 3, we have plotted the temperature (T) dependence of magnetization (M) for (C6H16N2) CoCl4 compound for different applied fields. The increase of magnetization is due to the orientation of spins in the same direction as the applied magnetic field. Note that the thermal evolution of the magnetization has no maximum that shows that the transition ferromagnetic–paramagnetic took place at the Neel temperature (T N) lower than 2 K. The magnetic properties are also shown in Fig. 4, in the form of \(\frac {1}{\chi }\) versus T plots. The low temperature magnetic susceptibility data was fit to the Curie–Weiss law, given by \(\chi =\frac {C}{\left ({T-\theta _{\text {cw}}} \right )}\). The experimental effective paramagnetic moments \(\mu _{\text {eff}}^{\exp }\) were calculated from \(C=\left ({\frac {N_{\mathrm {A}}}{3K_{\mathrm {B}}}}\right )\mu _{\text {eff}}^{2}\), where C, 𝜃 cw, N A, K B, and μ B have their usual meanings. The values of C, 𝜃 cw, and \(\mu _{\text {eff}}^{\exp }\) for the applied magnetic fields are deduced from Fig. 4 and are tabulated in Table 4. On the other hand, the theoretical effective moment can be calculated as follows [13]:
In our case, according to our sample which contains one unpaired electron Co(II) (3d 7, S= 5/2), we found that the value of the effective moment (\(\mu _{\text {eff}}^{\text {the}})\) is the order of 5.91 μ B. Such a difference between the experimental effective moment and the theoretical one can be explained by the existence of shortrange antiferromagnetic correlations in the paramagnetic state [14, 15]. The negative temperatures intercept together with the decrease of the effective magnetic moment and are in agreement with the existence of an antiferromagnetic exchange.
Table 4 shows the variation of experimental effective moment as a function of magnetic field. The observed decrease in effective magnetic moment is due to the spin–orbit coupling [2] The similar behavior is observed by Issaoui et al. [4]. To investigate other magnetic effects, the variation of M vs μ 0 H were carried out and the results indicate that the compound possesses a hysteretic behavior (Fig. 5). This hysteresis is consistent with the observed deviation of the Curie–Weiss law (Fig. 4) since a weak ferromagnetic ordering at low temperatures takes place.
Table 5 summarizes the obtained results from the calculation of the exchange parameter (J/ k B) in order to estimate the order of the exchange interaction between the neighboring cobalt ions using the following equation: \(\theta _{\text {cw}} =2Z(S(S+1))/3\left ({J/K_{\mathrm {B}}} \right )\).
The large values of J/k B are suggestive of an antiferromagnetically coupled system with an S= 3/2 ground state between the neighboring cobalt ions as expected from the 1/ χ versus T plots as well as from the structural data.
4 Conclusion
Treatment of 1-(2aminoethyl) pyrrolidine (C 6 H 14 N 2) with CoCl 2⋅6H 2O in aqueous solution gives the organic–inorganic hybrid ionic compound (C6H16N2) CoCl4 The asymmetric unit of (C6H16N2) CoCl4 is constituted by isolated monomeric tetrachloro cobaltat(II) and independent 1-(2aminoethyl) pyrrolidinium cations linked via N–H…Cl hydrogen bonds. The compound crystallizes at room temperature in the orthorhombic space group P2 12121.
Magnetic measurements revealed that the thermal variation of the magnetic susceptibility exhibited an antiferromagnetic interaction between the Co(II) centers which has been confirmed by the values of the exchange parameter. The isothermal magnetization curves with the absence of magnetic hysteresis loops proved the perfect magnetic reversibility of (C6H16N2) CoCl4 compound.
References
Long, G.S., Wei, M., Willett, R.D.: Inorg. Chem. 36(14), 3102– 3107 (1997)
Baccar, I., Issaoui, F., Zouari, F., Hussein, M., Dhahri, E., Valente, M.A.: Solid State Commun. 150, 2005–2010 (2010)
Parent, A.R., Landee, C.P., Turnbull, M.M.: Inorg. Chim. Acta 360, 1943–1953 (2007)
Issaoui, F., Baccar, I., Dhahri, E., El Sadek, O., Zouari, F., Hlil, E.K.: J. Supercond. Nov. Magn. 25, 1563–1570 (2012)
Luque, A., Sertucha, J., Castillo, O., Román, P.: Polyhedron 21, 19–26 (2002)
Ciampolini, M., Nardi, N., Vantacoli, B., Micheloni, M.: Coord. Chem. Rev. 120, 223 (1992)
Mitzi, D.B.: Prog. Inorg. Chem. 48, 1–4 (1999). and references therein
Carlin, R.L.: J. Am. Chem. Soc. 83, 3773 (1961)
Parent, A.R., Landee, C.P., Turnbull, M.M.: Inorg. Chim. Acta 360, 1943–1953 (2007)
Sheldrick, G.M.: Acta Crystallogr. Sect. A 64, 112 (2008)
Jebas, S.R., Balasubramanian, T., Light, M.E.: Acta Crystallogr. E 62, m1818—m1819 (2006)
Zhang, H., Fang, L., Yuan, R.: Acta Crystallogr. E 61, m677—m678 (2005)
Kittel, C.: Introduction to Solid State Physics, 6th, pp 404–406. Wiley, New York (1985)
Terashita, H., Neumeier, J.J.: Phys. Rev. B 71, 134 (2005)
Zemni, S., Gasmi, A., Boudard, M., Oumezzine, M.: Mater. Sci. Eng. B 144, 117 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Issaoui, F., Baklouti, Y., Dhahri, E. et al. Crystal Structure and Magnetic Property Studies of a Novel Hybrid Compound (C6H16N2) CoCl4 . J Supercond Nov Magn 28, 2621–2626 (2015). https://doi.org/10.1007/s10948-015-3057-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-015-3057-y