Abstract
Purpose
The objective of this study was to demonstrate that nontuberculous mycobacteria (NTM) pulmonary infections are not so infrequent and that the diagnosis may be suggested on the basis of the high-resolution computed tomography (HRCT) pattern alone.
Materials and methods
We retrospectively reviewed HRCT scans of 29 patients (9 men, 18 women; mean age 63 years, range 38–88 years) with positive culture from bronchial wash. Mycobacterium avium complex (MAC) was present in all (with the exception of one in whom the NTM was indistinct). In six patients, MAC was associated with M. chelonae, M. kansasii, M. fortuitum or M. xenopi. In one of these patients, MAC was associated with both M. fortuitum and M. chelonae. All patients had had nonspecific symptoms of pulmonary infection for a time ranging from 6 months to 12 years. Previous tuberculous infection was present in five patients (18.5%). Eleven patients had other pulmonary diseases (40.8%), and 12 had associated systemic diseases (44.4%).
Results
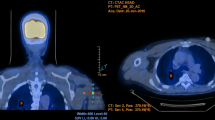
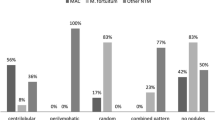
HRCT findings were apical fibrotic scarring (n=8; 29.6%), consolidations (n=16; 59.2%), single/multiple nodules >1 cm (n=8, multiple; 29.6%), cavitations (n=7; 25.9%), ground glass appearance (n=3; 11.1%), reticular/reticulonodular pattern (n=6; 22.2%), bronchiectasis (n=25; 92.5%), centrilobular nodules (tree in bud) (n=24; 88.8%), air trapping (n=8; 29.6%), lymphadenopathy >1 cm, also with calcification (n=13, 3 with calcification; 48.1%) and pleural effusion (n=2; 7.4%). In 3/7 patients with nodules >1 cm and with cavitations, the “feeding bronchus sign” (a patent bronchus running into a cavitation) was present. Lesions were in the upper lobes in 23 (85.1%), middle lobe/lingula in 25 (92.5%) and lower lobes in 18 (66.6%) patients. The findings were diffuse in 13 (48.1%) cases and patchy in 17 (62.9%).
Conclusions
HRCT findings are essential for the diagnosis of NTM pulmonary infection. The presence of bronchiectasis, cavitary nodules with feeding bronchus sign and tree-in-bud nodules in the middle lobe and lingula are suggestive of NTM infection, thus assisting the physician in the diagnostic workup of these patients.
Riassunto
Obiettivo
Scopo di questo lavoro è di dimostrare come l’infezione da micobatteri non tubercolari (NTM) non sia così infrequente e come sia possibile suggerirla al clinico e al microbiologo, a volte solo sulla base del pattern in tomografia computerizzata ad alta risoluzione (HRCT).
Materiali e metodi
Sono stati rivalutati retrospettivamente gli esami HRCT di 27 pazienti (9 maschi e 18 femmine; età media 63 anni, range 38–88 anni) con diagnosi microbiologica (esame colturale del broncoaspirato) di infezione da NTM. In tutti i pazienti era presente il M. avium complex (MAC), tranne in uno in cui il NTM è rimasto indefinito; in 6 pazienti vi era associazione di MAC con M. chelone, M. kansasii, M. fortuitum o M. xenopi. In uno di questi vi era l’associazione di MAC sia con M. fortuitum che M. chelone. In tutti erano presenti sintomi aspecifici di patologia polmonare (dispnea, tosse, a volte con escreato, febbre o febbricola, calo ponderale e astenia) con durata variabile da 6 mesi a 12 anni. Pregressa infezione tubercolare era presente in 5 pazienti (18,5%), 11 pazienti (40,8%) avevano fattori di rischio rappresentati da pre-esistenti malattie polmonari e 12 (44,4%) da malattie sistemiche, anche in associazione tra loro.
Risultati
I segni HRCT riscontrati sono stati esiti cicatriziali agli apici (8, 29,6%), addensamenti parenchimali (16, 59,2%), nodulo solitario/multipli con diametro >1 cm (8 multipli, 29,6%), cavitazioni dei noduli (7/8, 87,5%), vetro smerigliato (3, 11,1%), quadro reticolare/reticolo-nodulare (6, 22,2%), bronchiettasie (25, 92,5%), noduli centrolobulari (albero con gemme) (24, 88,8%), air trapping (8, 29,6%), linfonodi con diametro >1 cm ed eventuali calcificazioni (13, 3 con calcificazioni, 48,1%), versamento pleurico (2, 7,4%). In 3 dei 7 pazienti con noduli maggiori di 1 cm e con cavitazione è stato individuato il feedings bronchus sign, cioè la presenza di un bronco dilatato con pareti ispessite, a contatto con il nodulo escavato. Le lesioni interessavano i lobi superiori in 23 (85,1%), i lobo medio/lingula in 25 (92,5%) e i lobi inferiori in 18 (66,6%) pazienti. La diffusione delle lesioni è stata uniforme in 13 (48,1%) casi e a carta geografica in 17 (62,9%).
Conclusioni
Si può affermare che l’HRCT è metodica fondamentale nel riconoscimento e nella diagnosi di infezione polmonare da NTM. La presenza di bronchiettasie, di noduli escavati con il feedings bronchus sign e di noduli centrolobulari ad albero con gemme, con distribuzione prevalente nel lobo medio e nella lingula devono suggerire la possibilità di infezione da NMT, e orientare così il clinico verso la ricerca del germe responsabile.
Similar content being viewed by others
References/Bibliografia
Dailloux M, Abalain ML, Laurain C et al (2006) Respiratory infections associated with nontubercolous mycobacteria in non-HIV patients. Eur Respir J 28:1211–1215
Waller EA, Roy A, Brumble L et al (2006) The expanding spectrum of Mycobacterium avium complexassociated pulmonary disease. Chest 130:1234–1241
Glassroth J (2008) Pulmonary disease due to nontuberculous mycobacteria. Chest 133:243–251
Reich JM, Johnson RE (1992) Mycobacterium avium complex disease presented as an isolated lingular or middle lobe pattern: The Lady Windermere Syndrome. Chest 101:1605–1609
Dhillon SS, Watanakunkorn C (2000) Lady Windermere syndrome: middle lobe bronchiettasis and Mycobacterium avium complex infection due to voluntary cough soppression. Clin Infect Dis 30:572–575
Geich JM, Johonson RE (1992) Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. The Lady Windermere syndrome. Chest 101:1605–1609
Prince DS, Peterson DD, Steiner RM et al (1989) Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med 321:863–868
Kuroishi S, Nakamura Y, Shirai M et al (2008) Mycobacterium avium complex disease: prognostic implication of highresolution computer tomography findings. Eur Respir J 32:147–152
Griffith DE, Aksamit T, Brown-Elliott BA et al (2007) An official ATS/IDSA statement: diagnosis, treatment and prevention of nontuberculous mycobacterial disease. Am J Respir Crit Care Med 175:367–416
Han D, Lee KS, Koh WJ et al (2003) Radiographic and CT findings of nontubercolous mycobacterial pulmonary infection caused by Mycobacterium abscessus. AJR Am J Roentgenol 181:513–517
Pedicelli G, Leonetti C, Valcarenghi L (2000) TBC e micobatteriosi nel paziente immunodepresso. Radiol Med 99(Suppl 2):281–286
Viterbo V, Midiri M, Stellacci G et al (2000) Sindrome da immunodeficienza acquisita e malattie polmonari da mycobacterium xenopi. Ruolo della tomografia computerizzata. Radiol Med 99:443–448
Wittram C, Weisbrod GL (2002) Mycobacterium avium complex lung disease in immunocompetent patients: radiography-CT correlation. Br J Radiol 75:340–344
Martinez S, McAdams HP, Batchu CS (2007) The many faces of pulmonary nontuberculous mycobacterial infection. AJR Am J Roentgenol 189:177–186
D’Arienzo P, Giampalma E, Lavecchia M et al (2002) Ruolo dell’HRCT nell’identificazione dei segni di micobatteriosi atipica polmonare. Radiol Med 103:158–170
Dodd JD, Souza CA, Muller NL (2006) Conventional high-resolution CT versus helical high-resolution MDCT in the detection of bronchiectasis. AJR Am J Roentgenol 187:414–420
Erasmus JJ, McAdams H, Farrell MA et al (1999) Pulmonary nontuberculous mybacterial infection: radiologic manifestations. Radiographics 19:1487–1503
Wallace RJ Jr, Zhang Y, Brown B et al (1998) Polyclonal mycobacterium avium complex infections in patients with nodular bronchiettasis. Am J Respir Crit Care Med 158:1235–1244
Patz EF, Swensen SJ, Erasmus J (1995) Pulmonary manifestations of nontuberculous mycobacterium. Radiol Clin North Am 33:719–729
Schonfeld N (2006) The mycobacterial mystery. Eur Respur J 28:1076–1078
Fowler SJ, French J, Screaton NJ et al (2006) Nontuberculous mycobacteria in bronchiectasis: prevalence and patient characteristics. Eur Respir J 28:1204–1210
Koh WJ, Lee KS, Kwnon OJ et al (2005) Bilateral bronchiettasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous mycobacterial pulmonary infection. Radiology 235:282–288
Wickremasinghe M, Ozerovitch LJ, Wodehouse T et al (2005) Nontuberculous mycobacteria in patients with bronchiectasis. Thorax 60:1045–1051
Kim TS, Koh WJ, Han J et al (2005) Hypothesis on the evolution of cavitary lesions in nontuberculous mycobacterial pulmonary infection: thin-section CT and histolopathologic correlation. AJR Am J Roentgenol 184:1247–1252
Jeong JY, Lee KS, Koh WJ et al (2004) Nontuberculous mycobacterial pulmonary infection in immunocompetent patients: comparison of thin-section CT and histopathologic findings. Radiology 231:880–886
Chung MJ, Lee KS, Koh WJ et al (2005) Thin-section CT findings of nontuberculous mycobacterial pulmonary diseases: comparison between Mycobacterium aviumintracellulare and Mycobacterium abscessus infection. J Korean Med Sci 20:777–783
Ferraro S, Cappabianca S, Brunese L et al (2009) HRCT in detection of pulmonary infections from nontuberculous mycobacteria: personal experience. Radiol Med 114:376–389
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Polverosi, R., Guarise, A., Balestro, E. et al. High-resolution CT of nontuberculous mycobacteria pulmonary infection in immunocompetent, non-HIV-positive patients. Radiol med 115, 191–204 (2010). https://doi.org/10.1007/s11547-009-0479-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-009-0479-2