Abstract
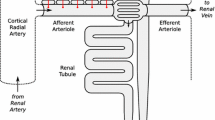
We have developed a mathematical model of the rat’s renal hemodynamics in the nephron level, and used that model to study flow control and signal transduction in the rat kidney. The model represents an afferent arteriole, glomerular filtration, and a segment of a short-loop nephron. The model afferent arteriole is myogenically active and represents smooth muscle membrane potential and electrical coupling. The myogenic mechanism is based on the assumption that the activity of nonselective cation channels is shifted by changes in transmural pressure, such that elevation in pressure induces vasoconstriction, which increases resistance to blood flow. From the afferent arteriole’s fluid delivery output, glomerular filtration rate is computed, based on conservation of plasma and plasma protein. Chloride concentration is then computed along the renal tubule based on solute conservation that represents water reabsorption along the proximal tubule and the water-permeable segment of the descending limb, and chloride fluxes driven by passive diffusion and active transport. The model’s autoregulatory response is predicted to maintain stable renal blood flow within a physiologic range of blood pressure values. Power spectra associated with time series predicted by the model reveal a prominent fundamental peak at ∼165 mHz arising from the afferent arteriole’s spontaneous vasomotion. Periodic external forcings interact with vasomotion to introduce heterodynes into the power spectra, significantly increasing their complexity.










Similar content being viewed by others
References
Chen, J., Sgouralis, I., Moore, L. C., Layton, H. E., & Layton, A. T. (2011). A mathematical model of the myogenic response to systolic pressure in the afferent arteriole. Am. J. Physiol., Ren. Fluid Electrolyte Physiol., 300, F669–F681.
Chon, K. H., Raghavan, R., Chen, Y.-M., Marsh, D. J., & Yip, K.-P. (2005). Interactions of TGF-dependent and myogenic oscillations in tubular pressure. Am. J. Physiol., Ren. Fluid Electrolyte Physiol., 288, F298–F307.
Cortell, S., Gennari, F. J., Davidman, M., Bossert, W. H., & Schwartz, W. B. (1973). A definition of proximal and distal tubular compliance. practical and theoretical implications. J. Clin. Invest., 52(9), 2330–2339.
Cupples, W. A., & Braam, B. (2007). Assessment of renal autoregulation. Am. J. Physiol., Ren. Fluid Electrolyte Physiol., 292(4), F1105–F1123.
Deen, W. M., Robertson, C. R., & Brenner, B. M. (1972). A model of glomerular ultrafiltration in the rat. Am. J. Physiol., 223(5), 1178–1183.
Fujii, K., Heistad, D. D., & Faraci, F. M. (1990). Ionic mechanisms in spontaneous vasomotion of the rat basilar artery in vivo. J. Physiol., 430(1), 389–398.
Gertz, K. H., Mangos, J. A., Braun, G., & Paget, H. D. (1965). On the glomerular tubular balance in the rat kidney. Pflügers Arch., 285, 360–372.
Gonzalez-Fernandez, J. M., & Ermentrout, B. (1994). On the origin and dynamics of the vasomotion of small arteries. Math. Biosci., 119, 127–167.
Holstein-Rathlou, N.-H., & Leyssac, P. P. (1986). TGF-mediated oscillations in the proximal intratubular pressure: differences between spontaneously hypertensive rats and Wistar-Kyoto rats. Acta Physiol. Scand., 126, 333–339.
Holstein-Rathlou, N.-H., & Marsh, D. J. (1994). Renal blood flow regulation and arterial pressure fluctuations: a case study in nonlinear dynamics. Physiol. Rev., 74, 637–681.
Holstein-Rathlou, N. H., & Marsh, D. J. (1989). Oscillations of tubular pressure, flow, and distal chloride concentration in rats. Am. J. Physiol., Ren. Fluid Electrolyte Physiol., 256(6), F1007–F1014.
Just, A. (2007). Mechanisms of renal blood flow autoregulation: dynamics and contributions. Am. J. Physiol., Regul. Integr. Comp. Physiol., 292, R1–17.
Just, A., & Arendshorst, W. J. (2002). Dynamics and contribution of mechanisms mediating renal blood flow autoregulation. Am. J. Physiol., Regul. Integr. Comp. Physiol., 285, R619–R631.
Just, A., Ehmke, H., Toktomambetova, L., & Kirchheim, H. R. (2001). Dynamic characteristics and underlying mechanisms of renal blood flow autoregulation in the conscious dog. Am. J. Physiol., Ren. Fluid Electrolyte Physiol., 280, F1062–F1071.
Layton, A. T. (2010). Feedback-mediated dynamics in a model of a compliant thick ascending limb. Math. Biosci., 228, 185–194.
Layton, A. T., Moore, L. C., & Layton, H. E. (2009). Tubuloglomerular feedback signal transduction in a compliant thick ascending limb. Am. J. Physiol., Ren. Fluid Electrolyte Physiol. doi:10.1152/ajprenal.00732.2010.
Layton, A. T., Pham, P., & Ryu, H. (2012). Signal transduction in a compliant short loop of Henle. Int. J. Numer. Methods Biomed. Eng., 28(3), 369–380.
Leyssac, P. P., & Holstein-Rathlou, N. H. (1986). Effects of various transport inhibitors on oscillating tgf pressure responses in the rat. Pflügers Arch., 407(3), 285–291.
Loutzenhiser, K., & Loutzenhiser, R. (2000). Angiotensin ii-induced ca2+ influx in renal afferent and efferent arterioles differing roles of voltage-gated and store-operated ca2+ entry. Circ. Res., 87(7), 551–557.
Loutzenhiser, R., Bidani, A., & Chilton, L. (2002). Renal myogenic response: kinetic attributes and physiologic role. Circ. Res., 90, 1316–1324.
Loutzenhiser, R., Bidani, A., & Wang, X. (2004). Systolic pressure and the myogenic response of the renal afferent arteriole. Acta Physiol. Scand., 181, 404–413.
Loutzenhiser, R., Griffin, K., Williamson, G., & Bidani, A. (2006). Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am. J. Physiol., Regul. Integr. Comp. Physiol., 290(5), R1153–R1167.
Lush, D. J., & Fray, J. C. S. (1984). Steady-state autoregulation of renal blood flow: a myogenic model. Am. J. Physiol., Regul. Integr. Comp. Physiol., 247, R89–R99.
Marsh, D. J., Sosnovtseva, O. V., Chon, K. H., & Holstein-Rathlou, N.-H. (2005). Nonlinear interactions in renal blood flow regulation. Am. J. Physiol., Regul. Integr. Comp. Physiol., 288, R1143–R1159.
Moore, L. C., Rich, A., & Casellas, D. (1994). Ascending myogenic autoregulation: interactions between tubuloglomerular feedback and myogenic mechanisms. Bull. Math. Biol., 56(3), 391–410.
Osol, G., & Halpern, W. (1988). Spontaneous vasomotion in pressurized cerebral arteries from genetically hypertensive rats. Am. J. Physiol., Heart Circ. Physiol., 254(1), H28–H33.
Raghavan, R., Chen, X., Yip, K.-P., Marsh, D. J., & Chon, K. H. (2006). Interactions between TGF-dependent and myogenic oscillations in tubular pressure and whole kidney blood flow in both SDR and SHR. Am. J. Physiol., Ren. Fluid Electrolyte Physiol., 290, F720–F732.
Schnermann, J., & Briggs, J. P. (2008). Function of the juxtaglomerular apparatus: control of glomerular hemodynamics and renin secretion. In R. J. Alpern & S. C. Hebert (Eds.), Seldin and Giebisch’s the Kidney: physiology and pathophysiology (4th ed., pp. 589–626). Amsterdam: Elsevier.
Sgouralis, I., & Layton, A. T. (2012). Autoregulation and conduction of vasomotor responses in a mathematical model of the rat afferent arteriole. Am. J. Physiol., Ren. Fluid Electrolyte Physiol. doi:10.1152/ajprenal.00589.2011.
Siegel, G., Ebeling, B. J., Hofer, H. W., Nolte, J., Roedel, H., & Klubendorf, D. (1984). Vascular smooth muscle rhythmicity. In Mechanisms of blood pressure waves (pp. 319–338).
Siu, K. L., Sung, B., Cupples, W. A., Moore, L. C., & Chon, K. H. (2009). Detection of low-frequency oscillations in renal blood flow. Am. J. Physiol., Ren. Fluid Electrolyte Physiol., 297, F155–F162.
Yip, K.-P., Holstein-Rathlou, N.-H., & Marsh, D. J. (1991). Chaos in blood flow control in genetic and renovascular hypertensive rats. Am. J. Physiol., Ren. Fluid Electrolyte Physiol., 261, F400–F408.
Acknowledgements
This research was supported, in part, by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, via grant DK089066.
Portions of this work were presented at Experimental Biology 2012 (FASEB J. 26:690.2, 2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sgouralis, I., Layton, A.T. Control and Modulation of Fluid Flow in the Rat Kidney. Bull Math Biol 75, 2551–2574 (2013). https://doi.org/10.1007/s11538-013-9907-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-013-9907-5