Abstract
Background
Diffuse large B-cell lymphoma (DLBCL) is a clinically heterogeneous malignancy. Following front-line immunochemotherapy, 30–40% of DLBCL patients develop relapsed or refractory (r/r) disease, which can be treated with ibrutinib. It has been previously reported that MYD88MUT affects the response to ibrutinib in patients with r/r DLBCL.
Objective
Here, we aimed to gather understanding of MYD88MUT in r/r DLBCL patients and to evaluate its influence on response to ibrutinib.
Patients and Methods
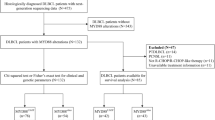
In this study, tissue samples from DLBCL patients (n = 212) were retrospectively collected and sequenced by target-capturing panels of either 413 or 112 genes that are frequently mutated in non-Hodgkin’s lymphoma. Sixty patients with MYD88 mutations and available clinical information were included for further analysis.
Results
Seven MYD88MUT variants were identified, L265P (65.0%, n = 39), S219C (13.3%, n = 8), S243N (8.3%, n = 5), P258L (6.7%, n = 4), F283V (1.7%, n = 1), P141R (1.7%, n = 1), and V217F (1.7%, n = 1). One patient had MYD88 amplification. In addition, mutations in PIM1 (67%, n = 40), IGH fusion (48%, n = 29), CD79B (43%, n = 26), KMT2D (30%, n = 18), and TP53 (27%, n = 17) were identified. For patients with L265P, IRF4 (p = 0.011) was frequently mutated. Otherwise, TET2 (p = 0.016), NOTCH2 (p = 0.044), MET (p = 0.037), SOCS1 (p = 0.011), TNFRSF14 (p = 0.011), EZH2 (p = 0.037), and BCL6 (p < 0.001) mutations were associated with MYD88MUT non-L265P variants. The incidence rate of MYD88MUT L265P was significantly higher with central nervous system involvement (p = 0.034). Four out of nine MYD88MUT patients responded to ibrutinib containing treatment, and this included those with MYD88MUT/CD79BWT.
Conclusions
This study adds clinical observations with MYD88MUT patients, further helping to understand the genetic features and possible correlation of MYD88MUT with response to ibrutinib.



Similar content being viewed by others
References
Yang QP, Zhang WY, Yu JB, Zhao S, Xu H, Wang WY, et al. Subtype distribution of lymphomas in Southwest China: analysis of 6,382 cases using WHO classification in a single institution. Diagn Pathol. 2011;6:77.
Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–8.
Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116(12):2040–5.
Cultrera JL, Dalia SM. Diffuse large B-cell lymphoma: current strategies and future directions. Cancer Control. 2012;19(3):204–13.
Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–11.
Bea S, Zettl A, Wright G, Salaverria I, Jehn P, Moreno V, et al. Diffuse large B-cell lymphoma subgroups have distinct genetic profiles that influence tumor biology and improve gene-expression-based survival prediction. Blood. 2005;106(9):3183–90.
Morin RD, Mungall K, Pleasance E, Mungall AJ, Goya R, Huff RD, et al. Mutational and structural analysis of diffuse large B-cell lymphoma using whole-genome sequencing. Blood. 2013;122(7):1256–65.
Dubois S, Jardin F. The role of next-generation sequencing in understanding the genomic basis of diffuse large B cell lymphoma and advancing targeted therapies. Expert Rev Hematol. 2016;9(3):255–69.
Burns K, Martinon F, Esslinger C, Pahl H, Schneider P, Bodmer JL, et al. MyD88, an adapter protein involved in interleukin-1 signaling. J Biol Chem. 1998;273(20):12203–9.
Dunne A, Ejdeback M, Ludidi PL, O'Neill LA, Gay NJ. Structural complementarity of Toll/interleukin-1 receptor domains in toll-like receptors and the adaptors Mal and MyD88. J Biol Chem. 2003;278(42):41443–51.
Muzio M, Ni J, Feng P, Dixit VM. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278(5343):1612–5.
Lee JH, Jeong H, Choi JW, Oh H, Kim YS. Clinicopathologic significance of MYD88 L265P mutation in diffuse large B-cell lymphoma: a meta-analysis. Sci Rep. 2017;7(1):1785.
Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470(7332):115–9.
Fernandez-Rodriguez C, Bellosillo B, Garcia-Garcia M, Sanchez-Gonzalez B, Gimeno E, Vela MC, et al. MYD88 (L265P) mutation is an independent prognostic factor for outcome in patients with diffuse large B-cell lymphoma. Leukemia. 2014;28(10):2104–6.
Rovira J, Karube K, Valera A, Colomer D, Enjuanes A, Colomo L, et al. MYD88 L265P mutations, but no other variants, identify a subpopulation of DLBCL patients of activated B-cell origin, extranodal involvement, and poor outcome. Clin Cancer Res. 2016;22(11):2755–64.
Dubois S, Viailly PJ, Bohers E, Bertrand P, Ruminy P, Marchand V, et al. Biological and clinical relevance of associated genomic alterations in MYD88 L265P and non-L265P-mutated diffuse large B-cell lymphoma: analysis of 361 cases. Clin Cancer Res. 2017;23(9):2232–44.
Yu S, Luo H, Pan M, Palomino LA, Song X, Wu P, et al. High frequency and prognostic value of MYD88 L265P mutation in diffuse large B-cell lymphoma with R-CHOP treatment. Oncol Lett. 2018;15(2):1707–15.
Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(8):922–6.
Winter AM, Landsburg DJ, Mato AR, Isaac K, Hernandez-Ilizaliturri FJ, Reddy N, et al. A multi-institutional outcomes analysis of patients with relapsed or refractory DLBCL treated with ibrutinib. Blood. 2017;130(14):1676–9.
Wang YL. MYD88 mutations and sensitivity to Ibrutinib therapy. J Mol Diagn. 2018;20(2):264–6.
Younes A, Sehn LH, Johnson P, Zinzani PL, Hong X, Zhu J, et al. Randomized phase III trial of Ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37(15):1285–95.
Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102(3):83–7.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–68.
Yang X, Chu Y, Zhang R, Han Y, Zhang L, Fu Y, et al. Technical validation of a next-generation sequencing assay for detecting clinically relevant levels of breast cancer-related single-nucleotide variants and copy number variants using simulated cell-free DNA. J Mol Diagn. 2017;19(4):525–36.
Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31(3):213–9.
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–303.
Li J, Lupat R, Amarasinghe KC, Thompson ER, Doyle MA, Ryland GL, et al. CONTRA: copy number analysis for targeted resequencing. Bioinformatics. 2012;28(10):1307–13.
Bohers E, Mareschal S, Bouzelfen A, Marchand V, Ruminy P, Maingonnat C, et al. Targetable activating mutations are very frequent in GCB and ABC diffuse large B-cell lymphoma. Genes Chromosomes Cancer. 2014;53(2):144–53.
Chapuy B, Roemer MG, Stewart C, Tan Y, Abo RP, Zhang L, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869–81.
Kraan W, Horlings HM, van Keimpema M, Schilder-Tol EJ, Oud ME, Scheepstra C, et al. High prevalence of oncogenic MYD88 and CD79B mutations in diffuse large B-cell lymphomas presenting at immune-privileged sites. Blood Cancer J. 2013;3:e139.
Morin RD, Mendez-Lago M, Mungall AJ, Goya R, Mungall KL, Corbett RD, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476(7360):298–303.
Pasqualucci L, Trifonov V, Fabbri G, Ma J, Rossi D, Chiarenza A, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet. 2011;43(9):830–7.
Zhang J, Grubor V, Love CL, Banerjee A, Richards KL, Mieczkowski PA, et al. Genetic heterogeneity of diffuse large B-cell lymphoma. Proc Natl Acad Sci USA. 2013;110(4):1398–403.
Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378(15):1396–407.
Chapuy B, Stewart C, Dunford AJ, Kim J, Kamburov A, Redd RA, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018;24(5):679–90.
Younes A, Brody J, Carpio C, Lopez-Guillermo A, Ben-Yehuda D, Ferhanoglu B, et al. Safety and activity of ibrutinib in combination with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: a phase 1/2a study. Lancet Haematol. 2019;6(2):e67–e78.
Acknowledgements
We thank all the patients, their families, and all the investigators who participated in the study.
Author information
Authors and Affiliations
Contributions
YKS, YQ, and SYJ conceptualized and designed the study. YKS, SYJ, YQ, LG, HXJ, BL, JLY, SY, XHH, and SYZ reviewed the literature, collected data, and interpreted the findings. SYJ, XHD, YTY, and JL performed statistical analysis. YKS, YQ, and SYJ drafted the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
This study was supported by the National Key Technology Support Program (2014BAI09B12), National New Drug Innovation Program (2017ZX09304015), Beijing-Tianjin-Hebei Cooperation Program for basic research under Grant H2018206591, and the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-1-001).
Conflict of Interest
Jing Lin is an employee of Burning Rock Biotech. Xinhua Du and Yuting Yi are employees of Geneplus-Beijing. Shiyu Jiang, Yan Qin, Lin Gui, Peng Liu, Hongxin Jiang, Biao Liu, Jianliang Yang, Sheng Yang, Xiaohui He, Shengyu Zhou, and Yuankai Shi declare that they have no conflicts of interest that might be relevant to the contents of this article.
Ethics Approval and Consent to Participate
The study was approved by the Review Board of Cancer Institute and Hospital, Chinese Academy of Medical Sciences and conducted in accordance with the Declaration of Helsinki. Consent to publish was obtained from the participants for reporting individual patient data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, S., Qin, Y., Gui, L. et al. Genomic Alterations and MYD88MUT Variant Mapping in Patients with Diffuse Large B-Cell Lymphoma and Response to Ibrutinib. Targ Oncol 15, 221–230 (2020). https://doi.org/10.1007/s11523-020-00710-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-020-00710-4