Abstract
Background
Data about the use and effectiveness of targeted therapy in metastatic small bowel adenocarcinoma (SBA) are scarce.
Objective
The aim of this population-based study was to obtain insights into the use and effectiveness of targeted therapy in patients with synchronous metastases of SBA.
Patients and methods
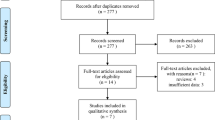
Data were retrieved from the Netherlands Cancer Registry. Patients treated with palliative chemotherapy and/or targeted therapy for synchronous metastatic SBA between 2007 and 2016 were included (n = 187). Differences in treatment and the subsequent effects on overall survival (OS) were evaluated.
Results
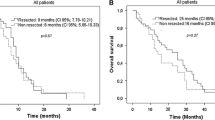
In first-line treatment, 25 patients (13%) received additional targeted therapy, exclusively bevacizumab, and mostly in combination with CAPOX/FOLFOX (n = 24). A primary ileal tumour was predictive for receiving bevacizumab in first-line treatment (odds ratio 3.2, 95% confidence interval (CI) 1.06–9.93). Median OS for patients in whom bevacizumab was added to first-line chemotherapy was 9.3 months, compared to 9.1 months with chemotherapy only (p = 0.85). Median OS for patients receiving first-line treatment only was 8.5 months with and 6.4 months without the addition of bevacizumab, respectively (p = 0.54). In multivariable survival analyses, the addition of bevacizumab was no prognostic factor (hazard ratio 1.01, 95% CI 0.65–1.59).
Conclusions
Bevacizumab was the only prescribed targeted therapy in first-line treatment. Considering the limited number of patients receiving first-line bevacizumab and the unknown reasons to prescribe additional targeted therapy, the corresponding survival rates of patients treated with and without additional bevacizumab in first-line treatment might suggest a limited clinical effect of bevacizumab in addition to first-line palliative chemotherapy on OS. Future research should focus on identifying the subgroup of patients who might benefit from anti-VEGF therapy in metastatic SBA.

Similar content being viewed by others
References
Zaanan A, Costes L, Gauthier M, et al. Chemotherapy of advanced small-bowel adenocarcinoma: a multicenter AGEO study. Ann Oncol. 2010;21(9):1786–93.
Aparicio T, Zaanan A, Svrcek M, et al. Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis. 2014;46(2):97–104.
Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249(1):63–71.
Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101(3):518–26.
Legue LM, Bernards N, Gerritse SL, et al. Trends in incidence, treatment and survival of small bowel adenocarcinomas between 1999 and 2013: a population-based study in The Netherlands. Acta Oncol. 2016;55(9–10):1183–9.
Overman MJ, Kopetz S, Wen S, et al. Chemotherapy with 5-fluorouracil and a platinum compound improves outcomes in metastatic small bowel adenocarcinoma. Cancer. 2008;113(8):2038–45.
Overman MJ, Varadhachary GR, Kopetz S, et al. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol. 2009;27(16):2598–603.
Tsushima T, Taguri M, Honma Y, et al. Multicenter retrospective study of 132 patients with unresectable small bowel adenocarcinoma treated with chemotherapy. Oncologist. 2012;17(9):1163–70.
Xiang XJ, Liu YW, Zhang L, et al. A phase II study of modified FOLFOX as first-line chemotherapy in advanced small bowel adenocarcinoma. Anticancer Drugs. 2012;23(5):561–6.
Zhang L, Wang LY, Deng YM, et al. Efficacy of the FOLFOX/CAPOX regimen for advanced small bowel adenocarcinoma: a three-center study from China. J Buon. 2011;16(4):689–96.
Aparicio T, Svrcek M, Zaanan A, et al. Small bowel adenocarcinoma phenotyping, a clinicobiological prognostic study. Br J Cancer. 2013;109(12):3057–66.
Kim TW, Elme A, Kusic Z, et al. A phase 3 trial evaluating panitumumab plus best supportive care vs best supportive care in chemorefractory wild-type KRAS or RAS metastatic colorectal cancer. Br J Cancer. 2016;115(10):1206–14.
Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–9.
Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25(13):1658–64.
Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28(31):4706–13.
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42.
Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23(16):3697–705.
Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–9.
Gulhati P, Raghav K, Shroff RT, et al. Bevacizumab combined with capecitabine and oxaliplatin in patients with advanced adenocarcinoma of the small bowel or ampulla of vater: A single-center, open-label, phase 2 study. Cancer. 2017;123(6):1011–7.
Hirao M, Komori M, Nishida T, et al. Clinical use of molecular targeted agents for primary small bowel adenocarcinoma: a multicenter retrospective cohort study by the Osaka Gut Forum. Oncol Lett. 2017;14(2):1628–36.
Takayoshi K, Kusaba H, Uenomachi M, et al. Suggestion of added value by bevacizumab to chemotherapy in patients with unresectable or recurrent small bowel cancer. Cancer Chemother Pharmacol. 2017;80(2):333–42.
Aydin D, Sendur MA, Kefeli U, et al. Evaluation of bevacizumab in advanced small bowel adenocarcinoma. Clin Colorectal Cancer. 2017;16(1):78–83.
Falcone R, Roberto M, Filetti M, Anselmi E, Marchetti P. Anti epidermal growth factor receptor therapy in small bowel adenocarcinoma: case report and literature review. Medicine (Baltimore). 2018;97(3):e9672.
Gulhati P, Raghav K, Shroff R, et al. Phase II study of panitumumab in RAS wild-type metastatic adenocarcinoma of small bowel or ampulla of vater. Oncologist. 2018;23(3):277-e26.
McWilliams RR, Foster NR, Mahoney MR, et al. North Central Cancer Treatment Group N0543 (Alliance): a phase 2 trial of pharmacogenetic-based dosing of irinotecan, oxaliplatin, and capecitabine as first-line therapy for patients with advanced small bowel adenocarcinoma. Cancer. 2017;123(18):3494–501.
Overman MJ, Pozadzides J, Kopetz S, et al. Immunophenotype and molecular characterisation of adenocarcinoma of the small intestine. Br J Cancer. 2010;102(1):144–50.
Acknowledgements
The authors thank the registration team of the Netherlands Cancer Registry for their dedicated data collection. We thank Nienke Bosman, Claudia van Leeuwen and Erica Masseling for their contribution to additional data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was financially supported by a grant from the Catharina Research Fund, which was used for additional data collection, and had no involvement in relation to the presented outcomes.
Conflict of interest
LL, FvE, NB, VL, IdH and GC declare that they have no conflicts of interest that might be relevant to the contents of this article.
Ethical approval
According to the Central Committee on Research involving Human Subjects (CCMO), this type of study does not require approval from an ethics committee.
Rights and permissions
About this article
Cite this article
Legué, L.M., van Erning, F.N., Bernards, N. et al. Addition of Bevacizumab to First-Line Palliative Chemotherapy in Patients with Metastatic Small Bowel Adenocarcinoma: A Population-Based Study. Targ Oncol 14, 699–705 (2019). https://doi.org/10.1007/s11523-019-00681-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-019-00681-1