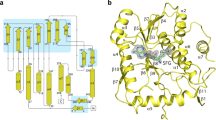
Abstract
Many flaviviruses are significant human pathogens. The plus-strand RNA genome of a flavivirus contains a 5′ terminal cap 1 structure (m7GpppAmG). The flavivirus encodes one methyltransferase (MTase), located at the N-terminal portion of the NS5 RNA-dependent RNA polymerase (RdRp). Here we review recent advances in our understanding of flaviviral capping machinery and the implications for drug development. The NS5 MTase catalyzes both guanine N7 and ribose 2′-OH methylations during viral cap formation. Representative flavivirus MTases, from dengue, yellow fever, and West Nile virus (WNV), sequentially generate GpppA → m7GpppA → m7GpppAm. Despite the existence of two distinct methylation activities, the crystal structures of flavivirus MTases showed a single binding site for S-adenosyl-L-methionine (SAM), the methyl donor. This finding indicates that the substrate GpppA-RNA must be repositioned to accept the N7 and 2′-O methyl groups from SAM during the sequential reactions. Further studies demonstrated that distinct RNA elements are required for the methylations of guanine N7 on the cap and of ribose 2′-OH on the first transcribed nucleotide. Mutant enzymes with different methylation defects can trans complement one another in vitro, demonstrating that separate molecules of the enzyme can independently catalyze the two cap methylations in vitro. In the context of the infectious virus, defects in both methylations, or a defect in the N7 methylation alone, are lethal to WNV. However, viruses defective solely in 2′-O methylation are attenuated and can protect mice from later wild-type WNV challenge. The results demonstrate that the N7 methylation activity is essential for the WNV life cycle and, thus, methyltransferase represents a novel and promising target for flavivirus therapy.
Similar content being viewed by others
References
Abraham G, Rhodes D P, Banerjee A K (1975). The 5′ terminal structure of the methylated mRNA synthesized in vitro by vesicular stomatitis virus. Cell, 5(1): 51–58
Ackermann M, Padmanabhan R (2001). De novo synthesis of RNA by the dengue virus RNA-dependent RNA polymerase exhibits temperature dependence at the initiation but not elongation phase. J Biol Chem, 276(43): 39926–39937
Ahola T, Kääriäinen L (1995). Reaction in alphavirus mRNA capping: formation of a covalent complex of nonstructural protein nsP1 with 7-methyl-GMP. Proc Natl Acad Sci U S A, 92(2): 507–511
Arias C F, Preugschat F, Strauss J H (1993). Dengue 2 virus NS2B and NS3 form a stable complex that can cleave NS3 within the helicase domain. Virology, 193(2): 888–899
Asnis D S, Conetta R, Teixeira A A, Waldman G, Sampson B A (2000). The West Nile Virus outbreak of 1999 in New York: the Flushing Hospital experience. Clin Infect Dis, 30(3): 413–418
Asnis D S, Conetta R, Waldman G, Teixeira A A (2001). The West Nile virus encephalitis outbreak in the United States (1999–2000): from Flushing, New York, to beyond its borders. Ann N Y Acad Sci, 951: 161–171
Assenberg R, Ren J, Verma A, Walter T S, Alderton D, Hurrelbrink R J, Fuller S D, Bressanelli S, Owens R J, Stuart D I, Grimes J M (2007). Crystal structure of the Murray Valley encephalitis virus NS5 methyltransferase domain in complex with cap analogues. J Gen Virol, 88(Pt 8): 2228–2236
Barbas C F 3rd, Heine A, Zhong G, Hoffmann T, Gramatikova S, Björnestedt R, List B, Anderson J, Stura E A, Wilson I A, Lerner R A (1997). Immune versus natural selection: antibody aldolases with enzymic rates but broader scope. Science, 278(5346): 2085–2092
Barbosa E, Moss B (1978). mRNA(nucleoside-2′-)-methyltransferase from vaccinia virus. Characteristics and substrate specificity. J Biol Chem, 253(21): 7698–7702
Benarroch D, Egloff M P, Mulard L, Guerreiro C, Romette J L, Canard B (2004). A structural basis for the inhibition of the NS5 dengue virus mRNA 2′-O-methyltransferase domain by ribavirin 5′-triphosphate. J Biol Chem, 279(34): 35638–35643
Bernard K A, Kramer L D (2001). West Nile virus activity in the United States, 2001. Viral Immunol, 14(4): 319–338
Bernard K A, Maffei J G, Jones S A, Kauffman E B, Ebel G, Dupuis A P 2nd, Ngo K A, Nicholas D C, Young D M, Shi P Y, Kulasekera V L, Eidson M, White D J, Stone W B, Kramer L D, and the NY State West Nile Virus Surveillance Team (2001). West Nile virus infection in birds and mosquitoes, New York State, 2000. Emerg Infect Dis, 7(4): 679–685
Bhattacharya D, Hoover S, Falk S P, Weisblum B, Vestling M, Striker R (2008). Phosphorylation of yellow fever virus NS5 alters methyltransferase activity. Virology, 380(2): 276–284
Bisaillon M, Lemay G (1997). Viral and cellular enzymes involved in synthesis of mRNA cap structure. Virology, 236(1): 1–7
Bollati M, Alvarez K, Assenberg R, Baronti C, Canard B, Cook S, Coutard B, Decroly E, de Lamballerie X, Gould E A, Grard G, Grimes J M, Hilgenfeld R, Jansson A M, Malet H, Mancini E J, Mastrangelo E, Mattevi A, Milani M, Moureau G, Neyts J, Owens R J, Ren J, Selisko B, Speroni S, Steuber H, Stuart D I, Unge T, Bolognesi M (2009a). Structure and functionality in flavivirus NSproteins: Perspectives for drug design. Antiviral Res, 2009 Nov 27. [Epub ahead of print] doi:10.1016/j.antiviral.2009.11.009
Bollati M, Milani M, Mastrangelo E, de Lamballerie X, Canard B, Bolognesi M (2009b). Crystal structure of a methyltransferase from a no-known-vector Flavivirus. Biochem Biophys Res Commun, 382(1): 200–204
Bollati M, Milani M, Mastrangelo E, Ricagno S, Tedeschi G, Nonnis S, Decroly E, Selisko B, de Lamballerie X, Coutard B, Canard B, Bolognesi M (2009c). Recognition of RNA cap in the Wesselsbron virus NS5 methyltransferase domain: implications for RNA-capping mechanisms in Flavivirus. J Mol Biol, 385(1): 140–152
Brinton M A (1981). Isolation of a replication-efficient mutant of West Nile virus from a persistently infected genetically resistant mouse cell culture. J Virol, 39(2): 413–421
Brinton M A (2002). The molecular biology of West Nile Virus: a new invader of the western hemisphere. Annu Rev Microbiol, 56: 371–402
Brinton M A, Dispoto J H (1988). Sequence and secondary structure analysis of the 5′-terminal region of flavivirus genome RNA. Virology, 162(2): 290–299
Burke D S, Monath T P (2001). Flaviviruses. Philadelphia, PA: Lippincott William & Wilkins
Centers for Disease Control and Prevention (CDC) (2000). Guidelines for surveillance, prevention, and control of West Nile virus infection—United States. MMWR Morb Mortal Wkly Rep, 49(2): 25–28
Chambers T J, Hahn C S, Galler R, Rice C M (1990). Flavivirus genome organization, expression, and replication. Annu Rev Microbiol, 44: 649–688
Chambers T J, Grakoui A, Rice C M (1991). Processing of the yellow fever virus nonstructural polyprotein: a catalytically active NS3 proteinase domain and NS2B are required for cleavages at dibasic sites. J Virol, 65(11): 6042–6050
Chambers T J, Nestorowicz A, Amberg S M, Rice C M (1993). Mutagenesis of the yellow fever virus NS2B protein: effects on proteolytic processing, NS2B-NS3 complex formation, and viral replication. J Virol, 67(11): 6797–6807
Chung K Y, Dong H, Chao A T, Shi P Y, Lescar J, Lim S P (2010). Higher catalytic efficiency of N-7-methylation is responsible for processive N-7 and 2′-O methyltransferase activity in dengue virus. Virology, 402(1): 52–60
Cleaves G R, Dubin D T (1979). Methylation status of intracellular dengue type 2 40 S RNA. Virology, 96(1): 159–165
Cong P, Shuman S (1992). Methyltransferase and subunit association domains of vaccinia virus mRNA capping enzyme. J Biol Chem, 267(23): 16424–16429
Davidson A D (2009). Chapter 2. New insights into flavivirus nonstructural protein 5. Adv Virus Res, 74: 41–101
De la Peña M, Kyrieleis O J, Cusack S (2007). Structural insights into the mechanism and evolution of the vaccinia virus mRNA cap N7 methyl-transferase. EMBO J, 26(23): 4913–4925
Diamond M S, Edgil D, Roberts T G, Lu B, Harris E (2000). Infection of human cells by dengue virus is modulated by different cell types and viral strains. J Virol, 74(17): 7814–7823
Dong H, Ray D, Ren S, Zhang B, Puig-Basagoiti F, Takagi Y, Ho C K, Li H, Shi P Y (2007). Distinct RNA elements confer specificity to flavivirus RNA cap methylation events. J Virol, 81(9): 4412–4421
Dong H, Ren S, Li H, Shi P Y (2008a). Separate molecules of West Nile virus methyltransferase can independently catalyze the N7 and 2′-O methylations of viral RNA cap. Virology, 377(1): 1–6
Dong H, Ren S, Zhang B, Zhou Y, Puig-Basagoiti F, Li H, Shi P Y (2008b). West Nile virus methyltransferase catalyzes two methylations of the viral RNA cap through a substrate-repositioning mechanism. J Virol, 82(9): 4295–4307
Dong H, Zhang B, Shi P Y (2008c). Flavivirus methyltransferase: a novel antiviral target. Antiviral Res, 80(1): 1–10
Egloff M P, Benarroch D, Selisko B, Romette J L, Canard B (2002). An RNA cap (nucleoside-2′-O-)-methyltransferase in the flavivirus RNA polymerase NS5: crystal structure and functional characterization. EMBO J, 21(11): 2757–2768
Egloff M P, Decroly E, Malet H, Selisko B, Benarroch D, Ferron F, Canard B (2007). Structural and functional analysis of methylation and 5′-RNA sequence requirements of short capped RNAs by the methyltransferase domain of dengue virus NS5. J Mol Biol, 372(3): 723–736
Fabrega C, Hausmann S, Shen V, Shuman S, Lima C D (2004). Structure and mechanism of mRNA cap (guanine-N7) methyltransferase. Mol Cell, 13(1): 77–89
Falgout B, Miller R H, Lai C J (1993). Deletion analysis of dengue virus type 4 nonstructural protein NS2B: identification of a domain required for NS2B-NS3 protease activity. J Virol, 67(4): 2034–2042
Fauman E B, Blumenthal R M, Cheng X D (1999) Structure and evolution of AdoMet-dependent methyltransferases. World Scientific Publishing Co., Singapore.
Fredericksen B L, Gale M Jr (2006). West Nile virus evades activation of interferon regulatory factor 3 through RIG-I-dependent and -independent pathways without antagonizing host defense signaling. J Virol, 80(6): 2913–2923
Frey P A, Kokesh F C, Westheimer F H (1971). A reporter group at the active site of acetoacetate decarboxylase. I. Ionization constant of the nitrophenol. J Am Chem Soc, 93(26): 7266–7269
Furuichi Y, Shatkin A J (2000). Viral and cellular mRNA capping: past and prospects. Adv Virus Res, 55: 135–184
Geiss B J, Thompson A A, Andrews A J, Sons R L, Gari H H, Keenan S M, Peersen O B (2009). Analysis of flavivirus NS5 methyltransferase cap binding. J Mol Biol, 385(5): 1643–1654
Gong W, O’Gara M, Blumenthal RM, Cheng X (1997). Structure of pvu II DNA-(cytosine N4) methyltransferase, an example of domain permutation and protein fold assignment. Nucleic Acids Res, 25(14): 2702–2715
Gu M, Lima C D (2005). Processing the message: structural insights into capping and decapping mRNA. Curr Opin Struct Biol, 15(1): 99–106
Guyatt K J, Westaway E G, Khromykh A A (2001). Expression and purification of enzymatically active recombinant RNA-dependent RNA polymerase (NS5) of the flavivirus Kunjin. J Virol Methods, 92(1): 37–44
Hager J, Staker B L, Bugl H, Jakob U (2002). Active site in RrmJ, a heat shock-induced methyltransferase. J Biol Chem, 277(44): 41978–41986
Highbarger L A, Gerlt J A, Kenyon G L (1996). Mechanism of the reaction catalyzed by acetoacetate decarboxylase. Importance of lysine 116 in determining the pKa of active-site lysine 115. Biochemistry, 35(1): 41–46
Hodel A E, Gershon P D, Shi X, Quiocho F A (1996). The 1.85 A structure of vaccinia protein VP39: a bifunctional enzyme that participates in the modification of both mRNA ends. Cell, 85(2): 247–256
Hodel A E, Gershon P D, Quiocho F A (1998). Structural basis for sequence-nonspecific recognition of 5′-capped mRNA by a capmodifying enzyme. Mol Cell, 1(3): 443–447
Hodel A E, Quiocho F A, Gershon P D (1999). VP39-an mRNA capspecific 2′-o-methyltransferase. In: X.D. Cheng and R.M. Blementhal, eds. S-Adenosylmethionine-dependent methyltransferase: structures and functions. 255–282
Hornung V, Ellegast J, Kim S, Brzózka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann K K, Schlee M, Endres S, Hartmann G (2006). 5′-Triphosphate RNA is the ligand for RIG-I. Science, 314(5801): 994–997
Horton J R, Sawada K, Nishibori M, Zhang X, Cheng X (2001). Two polymorphic forms of human histamine methyltransferase: structural, thermal, and kinetic comparisons. Structure, 9(9): 837–849
Issur M, Geiss B J, Bougie I, Picard-Jean F, Despins S, Mayette J, Hobdey S E, Bisaillon M (2009). The flavivirus NS5 protein is a true RNA guanylyltransferase that catalyzes a two-step reaction to form the RNA cap structure. RNA, 15(12): 2340–2350
Jansson A M, Jakobsson E, Johansson P, Lantez V, Coutard B, de Lamballerie X, Unge T, Jones T A (2009). Structure of the methyltransferase domain from the Modoc virus, a flavivirus with no known vector. Acta Crystallogr D Biol Crystallogr, 65(Pt 8): 796–803
Kamer G, Argos P (1984). Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res, 12(18): 7269–7282
Khromykh A A, Kenney M T, Westaway E G (1998). Transcomplementation of flavivirus RNA polymerase gene NS5 by using Kunjin virus replicon-expressing BHK cells. J Virol, 72(9): 7270–7279
Kokesh F C, Westheimer F H (1971). A reporter group at the active site of acetoacetate decarboxylase. II. Ionization constant of the amino group. J Am Chem Soc, 93(26): 7270–7274
Komoto J, Huang Y, Takata Y, Yamada T, Konishi K, Ogawa H, Gomi T, Fujioka M, Takusagawa F (2002). Crystal structure of guanidinoacetate methyltransferase from rat liver: a model structure of protein arginine methyltransferase. J Mol Biol, 320(2): 223–235
Koonin E V (1991). The phylogeny of RNA-dependent RNA polymerases of positive-strand RNA viruses. J Gen Virol, 72(Pt 9): 2197–2206
Koonin E V (1993). Computer-assisted identification of a putative methyltransferase domain in NS5 protein of flaviviruses and lambda 2 protein of reovirus. J Gen Virol, 74(Pt 4): 733–740
Kramer L D, Bernard K A (2001). West Nile virus infection in birds and mammals. Ann N Y Acad Sci, 951: 84–93
Kramer L D, Li J, Shi P Y (2007). West Nile virus. Lancet Neurol, 6(2): 171–181
Kroschewski H, Lim S P, Butcher R E, Yap T L, Lescar J, Wright P J, Vasudevan S G, Davidson A D (2008). Mutagenesis of the dengue virus type 2 NS5 methyltransferase domain. J Biol Chem, 283(28): 19410–19421
Kümmerer B M, Rice C M (2002). Mutations in the yellow fever virus nonstructural protein NS2A selectively block production of infectious particles. J Virol, 76(10): 4773–4784
Kwon T, Chang J H, Kwak E, Lee C W, Joachimiak A, Kim Y C, Lee J, Cho Y (2003). Mechanism of histone lysine methyl transfer revealed by the structure of SET7/9-AdoMet. EMBO J, 22(2): 292–303
Li H, Clum S, You S, Ebner K E, Padmanabhan R (1999). The serine protease and RNA-stimulated nucleoside triphosphatase and RNA helicase functional domains of dengue virus type 2 NS3 converge within a region of 20 amino acids. J Virol, 73(4): 3108–3116
Li J, Wang J T, Whelan S P (2006). A unique strategy for mRNA cap methylation used by vesicular stomatitis virus. Proc Natl Acad Sci U S A, 103(22): 8493–8498
Li L, Lok S M, Yu I M, Zhang Y, Kuhn R J, Chen J, Rossmann M G (2008). The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science, 319(5871): 1830–1834
Lim S P, Wen D, Yap T L, Yan C K, Lescar J, Vasudevan S G (2008). A scintillation proximity assay for dengue virus NS5 2′-O-methyltransferase-kinetic and inhibition analyses. Antiviral Res, 80(3): 360–369
Lindenbach B D, Rice C M (1997). trans-Complementation of yellow fever virus NS1 reveals a role in early RNA replication. J Virol, 71(12): 9608–9617
Lindenbach B D, Rice C M (1999). Genetic interaction of flavivirus nonstructural proteins NS1 and NS4A as a determinant of replicase function. J Virol, 73(6): 4611–4621
Luzhkov V B, Selisko B, Nordqvist A, Peyrane F, Decroly E, Alvarez K, Karlen A, Canard B, Qvist J (2007). Virtual screening and bioassay study of novel inhibitors for dengue virus mRNA cap (nucleoside-2′O)-methyltransferase. Bioorg Med Chem, 15(24): 7795–7802
Malone T, Blumenthal R M, Cheng X (1995). Structure-guided analysis reveals nine sequence motifs conserved among DNA amino-methyltransferases, and suggests a catalytic mechanism for these enzymes. J Mol Biol, 253(4): 618–632
Martin J L, McMillan F M (2002). SAM (dependent) I AM: the S-adenosylmethionine-dependent methyltransferase fold. Curr Opin Struct Biol, 12(6): 783–793
Mastrangelo E, Bollati M, Milani M, Selisko B, Peyrane F, Canard B, Grard G, de Lamballerie X, Bolognesi M (2007). Structural bases for substrate recognition and activity in Meaban virus nucleoside-2′-Omethyltransferase. Protein Sci, 16(6): 1133–1145
Milani M, Mastrangelo E, Bollati M, Selisko B, Decroly E, Bouvet M, Canard B, Bolognesi M (2009). Flaviviral methyltransferase/RNA interaction: structural basis for enzyme inhibition. Antiviral Res, 83(1): 28–34
Moure C M, Bowman B R, Gershon P D, Quiocho F A (2006). Crystal structures of the vaccinia virus polyadenylate polymerase heterodimer: insights into ATP selectivity and processivity. Mol Cell, 22(3): 339–349
Muylaert I R, Chambers T J, Galler R, Rice C M (1996). Mutagenesis of the N-linked glycosylation sites of the yellow fever virus NS1 protein: effects on virus replication and mouse neurovirulence. Virology, 222(1): 159–168
Muylaert I R, Galler R, Rice C M (1997). Genetic analysis of the yellow fever virus NS1 protein: identification of a temperature-sensitive mutation which blocks RNA accumulation. J Virol, 71(1): 291–298
Nicholls A, Sharp K A, Honig B (1991). Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins, 11(4): 281–296
Ogino T, Banerjee A K (2007). Unconventional mechanism of mRNA capping by the RNA-dependent RNA polymerase of vesicular stomatitis virus. Mol Cell, 25(1): 85–97
Perera R, Kuhn R J (2008). Structural proteomics of dengue virus. Curr Opin Microbiol, 11(4): 369–377
Petersen L R, Roehrig J T (2001). West Nile virus: a reemerging global pathogen. Emerg Infect Dis, 7(4): 611–614
Peyrane F, Selisko B, Decroly E, Vasseur J J, Benarroch D, Canard B, Alvarez K (2007). High-yield production of short GpppA- and 7MeGpppA-capped RNAs and HPLC-monitoring of methyltransfer reactions at the guanine-N7 and adenosine-2′O positions. Nucleic Acids Res, 35(4): e26
Podvinec M, Lim S P, Schmidt T, Scarsi M, Wen D, Sonntag L S, Sanschagrin P, Shenkin P S, Schwede T (2010). Novel inhibitors of dengue virus methyltransferase: discovery by in vitro-driven virtual screening on a desktop computer grid. JMed Chem, 53(4): 1483–1495
Puig-Basagoiti F, Qing M, Dong H, Zhang B, Zou G, Yuan Z, Shi P Y (2009). Identification and characterization of inhibitors of West Nile virus. Antiviral Res, 83(1): 71–79
Ray D, Shah A, Tilgner M, Guo Y, Zhao Y, Dong H, Deas T S, Zhou Y, Li H, Shi P Y (2006). West Nile virus 5′-cap structure is formed by sequential guanine N-7 and ribose 2′-O methylations by nonstructural protein 5. J Virol, 80(17): 8362–8370
Reinisch K M, Nibert M L, Harrison S C (2000). Structure of the reovirus core at 3.6 A resolution. Nature, 404(6781): 960–967
Rice C M, Lenches E M, Eddy S R, Shin S J, Sheets R L, Strauss J H (1985). Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science, 229(4715): 726–733
Sampath A, Padmanabhan R (2009). Molecular targets for flavivirus drug discovery. Antiviral Res, 81(1): 6–15
Schnierle B S, Gershon P D, Moss B (1994). Mutational analysis of a multifunctional protein, with mRNA 5′ cap-specific (nucleoside-2′-O-)-methyltransferase and 3′-adenylyltransferase stimulatory activities, encoded by vaccinia virus. J Biol Chem, 269(32): 20700–20706
Selisko B, Peyrane F F, Canard B, Alvarez K, Decroly E (2010). Biochemical characterization of the (nucleoside-2′O)-methyltransferase activity of dengue virus protein NS5 using purified capped RNA oligonucleotides (7Me)GpppAC(n) and GpppAC(n). J Gen Virol, 91(Pt 1): 112–121
Shi P Y, Kauffman E B, Ren P, Felton A, Tai J H, Dupuis A P 2nd, Jones S A, Ngo K A, Nicholas D C, Maffei J, Ebel G D, Bernard K A, Kramer L D (2001). High-throughput detection of West Nile virus RNA. J Clin Microbiol, 39(4): 1264–1271
Shi P Y, Tilgner M, Lo M K (2002a). Construction and characterization of subgenomic replicons of New York strain of West Nile virus. Virology, 296(2): 219–233
Shi P Y, Tilgner M, Lo MK, Kent K A, Bernard K A (2002b). Infectious cDNA clone of the epidemic west nile virus from New York City. J Virol, 76(12): 5847–5856
Shiryaev S A, Ratnikov B I, Chekanov A V, Sikora S, Rozanov D V, Godzik A, Wang J, Smith J W, Huang Z, Lindberg I, Samuel M A, Diamond M S, Strongin A Y (2006). Cleavage targets and the D-arginine-based inhibitors of the West Nile virus NS3 processing proteinase. Biochem J, 393(Pt 2): 503–511
Shuman S (2001). Structure, mechanism, and evolution of the mRNA capping apparatus. Prog Nucleic Acid Res Mol Biol, 66: 1–40
Smithburn B C, Hughes T P, Burke AW, Paul J H (1940). A neurotropic virus isolated from the blood of a native Uganda. Am J Trop Med Hyg, 20: 471–492
Sutton G, Grimes JM, Stuart D I, Roy P (2007). Bluetongue virus VP4 is an RNA-capping assembly line. Nat Struct Mol Biol, 14(5): 449–451
Tan B H, Fu J, Sugrue R J, Yap E H, Chan Y C, Tan Y H (1996). Recombinant dengue type 1 virus NS5 protein expressed in Escherichia coli exhibits RNA-dependent RNA polymerase activity. Virology, 216(2): 317–325
Warrener P, Tamura J K, Collett M S (1993). RNA-stimulated NTPase activity associated with yellow fever virus NS3 protein expressed in bacteria. J Virol, 67(2): 989–996
Wengler G, Wengler G (1981). Terminal sequences of the genome and replicative-from RNA of the flavivirus West Nile virus: absence of poly(A) and possible role in RNA replication. Virology, 113(2): 544–555
Wengler G, Wengler G (1991). The carboxy-terminal part of the NS 3 protein of theWest Nile flavivirus can be isolated as a soluble protein after proteolytic cleavage and represents an RNA-stimulated NTPase. Virology, 184(2): 707–715
Westaway E G, Brinton MA, Gaidamovich S Y, Horzinek MC, Igarashi A, Kaariainen L, Lvov D K, Porterfield J S, Russell P K, Trent D W (1985). Flaviviridae Intervirol, 24: 183–192
WHO (2009a). Dengue factsheet. http://www.who.int/mediacentre/factsheets/fs117/en/
WHO (2009b). Immunization, vaccines and biologicals: Japanese encephalitis. http://www.who.int/nuvi/je/en/
WHO (2009c). Yellow fever factsheet. http://www.who.int/mediacentre/factsheets/fs100/en/
Yu I M, Zhang W, Holdaway H A, Li L, Kostyuchenko V A, Chipman P R, Kuhn R J, Rossmann M G, Chen J (2008). Structure of the immature dengue virus at low pH primes proteolytic maturation. Science, 319(5871): 1834–1837
Zhang X, Zhou L, Cheng X (2000). Crystal structure of the conserved core of protein arginine methyltransferase PRMT3. EMBO J, 19(14): 3509–3519
Zhou Y, Ray D, Zhao Y, Dong H, Ren S, Li Z, Guo Y, Bernard K A, Shi P Y, Li H (2007). Structure and function of flavivirus NS5 methyltransferase. J Virol, 81(8): 3891–3903
Zubieta C, He X Z, Dixon R A, Noel J P (2001). Structures of two natural product methyltransferases reveal the basis for substrate specificity in plant O-methyltransferases. Nat Struct Biol, 8(3): 271–279
Zuker M (2003). Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res, 31(13): 3406–3415
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Liu, L., Dong, H., Chen, H. et al. Flavivirus RNA cap methyltransferase: structure, function, and inhibition. Front. Biol. 5, 286–303 (2010). https://doi.org/10.1007/s11515-010-0660-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11515-010-0660-y