Abstract
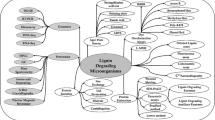
Lignin is both the most abundant aromatic (phenolic) polymer and the second most abundant raw material. It is degraded and modified by bacteria in the natural world, and bacteria seem to play a leading role in decomposing lignin in aquatic ecosystems. Lignin-degrading bacteria approach the polymer by mechanisms such as tunneling, erosion, and cavitation. With the advantages of immense environmental adaptability and biochemical versatility, bacteria deserve to be studied for their ligninolytic potential.
Similar content being viewed by others
References
Adeiran S A, Lambeir A M (1989). Kinetics of the reaction of compound II of horseradish peroxidase with hydrogen peroxide to form compound III. European Journal of Biochemistry, 186: 571
Ajit V, Bala K, Jaishree P, Shailendra S, Helmut K (1994). Lignocellulose degradation by microorganisms from termite hills and termite guts: A survey on the present state of art. FEMS Microbiology Review, 15: 9–28
Alexandre G, Zhulin I B (2000). Laccases are widespread in bacteria. Trends in Biotechnology, 18: 41–42
Alfred M M (2006). Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry, 67: 2318–2331
Antai S P, Crawford D L (1981). Degradation of softwood, hardwood and grass lignocellulose by two Streptomyces strains. Applied and Environment Microbiology, 42: 378–380
Archana P I, Mahadevan A (2002). Lignin degradation by bacteria. Progress in Industrial Microbiology, 36: 311–330
Argyropoulos D S, Menachem S B (1997). Lignin. In: Eriksson K E L, eds. Advances in Biochemical Engineering Biotechnology. vol 57. Germany: Springer, 127–158
Balba M T, Evans W C (1977). The methanogenic fermentation of aromatic substrates. Biochemical Society Transactions, 5: 302–303
Baldrian P, Gabriel J (2002). Copper and cadmium increase laccase activity in Peurotus ostreatus. FEMS Microbiology Letters, 206: 69–74
Barreca A M, Sjögren B, Fabbrini M, Galli C, Gentili P (2004). Catalytic efficiency of some mediators in laccase-catalyzed alcohol oxidation. Biocatalysis and Biotransformation, 22: 105–112
Benner R, Maccubbin A E, Hodson R E (1984). Anaerobic biodegradation of the lignin and polysaccharide components of lignocellulose and synthetic lignin by sediment microflora. Applied and Environment Microbiology, 47:998–1004
Biegert T, Altenschmidt U, Eckerskorn C, Fuchs G (1993). Enzymes of anaerobic metabolism of phenolic compounds 4-hydroxybenzoate-CoA ligase from a denitrifying pseudomonas species. European Journal of Biochemistry, 213: 555–561
Björdal C G, Nilsson T, Daniel G (1999). Microbial decay of waterlogged archaeological wood found in Sweden Applicable to archaeology and conservation. International Biodeterioration & Biodegradation, 43: 63–73
Blanchette R A (1995). Degradation of the lignocellulose complex in wood. Canadian Journal of Botany, 73 (1): 999–1010
Boll M, Albracht S J P, Fuchs G (1997). Benzoyl-CoA reductase (dearomatizing), a key enzyme of anaerobic aromatic metabolism. A study of adenosinephosphate activity, ATP stoichiometry of the reaction and EPR properties of the enzyme. European Journal of Biochemistry, 244: 840–851
Boll M, Fuchs G (1995). Benzoyl-coenzyme A reductase (dearomatizing), a key enzyme of anaerobic aromatic metabolism. ATP dependence of the reaction, purification and some properties of the enzyme from Thauera aromatica strain K172. European Journal of Biochemistry, 234: 921–933
Boll, M, Laempe, D, Eisenreich, W, Bacher, A, Mittelberger, T, Heinze, J, Fuchs, G (2000). Non-aromatic products from anoxic conversion of benzoyl-CoA with benzoyl-CoA reductase and cyclohexa-1, 5-diene-1-carbonyl-CoA hydratase. The Journal of Biological Chemistry, 275: 21889–21895
Bourbonnais, R, Paice, M G, Freiermuth, B, Bodie, E, Borneman, S (1997). Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Applied and Environment Microbiology, 63: 4627–4632
Brackmann, R, Fuchs, G (1993). Enzymes of anaerobic metabolism of phenolic compounds— 4-hydroxybenzoyl-CoA reductase (dehydroxylating) from a denitrifying Pseudomonas species. European Journal of Biochemistry, 213: 563–571
Brunow G (2001). Methods to reveal the structure of lignin. In: Steinbüchel A, Hofrichter M, eds. Biopolymers. Vol 1—Lignin, humic substances and coal. Weinheim, Germany: Wiley-VCH, 89–116
Chandra, R, Raj, A, Purohit, H J, Kapley, A (2007). Characterization and optimization of three potential aerobic bacterial strains for kraft lignin degradation from pulp paper waste. Chemosphere, 67 (4): 839–846
Claus, H, Faber, G, König, H (2002). Redox-mediated decolorization of synthetic dyes by fungal laccases. Applied Microbiology and Biotechnology, 59: 672–678
Crawford, D L, Sutherland, J B, Pometto, A L, Miller, J M (1982). Production of an aromatic aldehyde oxidase by Streptomyees viridosporus. Archives of Microbiology, 131: 140–145
Crawford, R L, Kirk, T K, Harkin, J M, McCoy, E (1973). Bacterial cleavage of an arylglycerol-β-aryl ether bond. Applied Microbiology, 25: 322–324
Crawford, R L, Robinson, L E, Cheh, A M (1980). 14C-labeled lignins as substrates for the study of lignin biodegradation and transformation. In: Kirk T K, Higuchi T, Chang H M, eds. Lignin Biodegradation: Microbiology Chemistry and Applications. USA: CRC Press, 61–76
Criquet, S, Joner, E, Leglize, P, Leyval, C (2000). Anthracene and mycorrhiza affect the activity of oxidoreductases in the roots and the rhizosphere of lucerne (Medicago sativa L.). Biotechnology Letters, 22: 1733–1737
Cullen, D, Kersten, P J (1996). Enzymology and molecular biology of lignin degradation. In: Brambl R, Marzluf G A, eds. The Mycota III, Biochemistry and Molecular Biology. Berlin: Springer-Verlag, 295–306
Daniel, G, Nilsson, T (1998). Developmennts in the study of soft rot and bacterial decay. In: Bruce A, Palfreyman J W, eds. Forest Products Biotechnology. London: Taylor & Francis
Dunford H B, Adeniran A J (1986). Hammett πσ correlation for reactions of horseradish peroxidase compound II with phenols. Archives of Biochemistry and Biophysics, 251: 536–542
Durán N (1997). The impact of biotechnology in the pulp and paper industry: state of art. Progress in microbial ecology (new trends and the application on biotechnology). Brazilian Soc Microbiol SBM/ICOME Publ Brazil, 543–549
Durán N, Esposito E (2000). Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: a review. Applied Catalysis. B. Environmental, 28: 83–99
Evans W C (1977). Biochemistry of the bacterial catabolism of aromatic compounds in anaerobic environments. Nature (London), 270: 17–22
Evans, W C, Fuchs, G (1988). Anaerobic degradation of aromatic compounds. Annual Review of Microbiology, 42: 289–317
Everse, J, Everse, K E, Grisham, M B (1991). Peroxidases in Chemistry and Biology. Vol 1 and 2, Boca Raton: CRC Press, 620
Farnet, A M, Criquet, S, Cigna, M, Gil, G, Ferre, E (2004). Purification of a laccase from Marasmius quercophilus induced with ferulic acid: reactivity towards natural and xenobiotic aromatic compounds. Enzyme and Microbial Technology, 34: 549–554
Finger, A (1994). In vitro studies on the effect of polyphenol oxidase and peroxidase on the formation of polyphenolics black tea c onstituents. Journal of the Science of Food and Agriculture, 66: 293–305
Forney, L J, Reddy, C A (1980). Bacterial degradation of Kraft lignin. Developments in Industrial Microbiology, 20: 163–175
Fukuzumi, T, Katayama, Y (1977). Bacterial degradation of dimer relating to structure of lignin. Mokuzai Gakkaishi, 23: 214–215
Galli, C, Gentili, P (2004). Chemical messengers: mediated oxidations with the enzyme laccase. Journal of Physical Organic Chemistry, 17: 973–977
Gianfreda, L, Rao, M A (2004). Potential of extra cellular enzymes in remediation of polluted soils: a review. Enzyme and Microbial Technology, 35: 339–354
Gibson, D T, Roberts, R L, Wells, M C, Cobal, V M (1973). Oxidation of biphenyl by a Beijerinckia species. Biochemical and Biophysical Research Communications, 50: 211–219
Gibson, J, Dispensa, M, Fogg, G C, Evans, D T, Harwood, C S (1994). 4-Hydroxybenzoate-coenzyme A ligase from Rhodopseudomonas palustris: purification, gene sequence, and role in anaerobic degradation. Journal of Bacteriology, 176: 634–641
Givaudan, A, Effosse, A, Faure, D, Potier, P, Bovillant, M, Bally, R (1993). Polyphenoloxidase from Azospirillum lipoferum isolated from the rhizosphere: evidence for a laccase in non-motile strains of Azospirillum lipoferum. FEMS Microbiology Letters, 108: 205–210
Gold, D M (1998). Identification, molecular cloning and DNA sequencing of the genes encoding protocatechuate 3,4-dioxygenase in Bacillus subtilis., University of Madras, Chennai, India
Hackett W F, Connors, W J, Kirk, T K, Zeikus, J G (1977). Microbial decomposition of synthetic 14C-labelled lignins in nature: Lignin biodegradation in a variety of natural materials. Applied and Environment Microbiology, 33: 43–51
Haemmerli, S D, Leisola, M S, Sanglard, D, Fiechter, A (1986). Oxidation of benzo(a)pyrene by extracellular ligninases of Phanerochaete chrysosporium. Veratryl alcohol and stability of ligninase. Journal of Biological Chemistry, 261: 6900–6903
Haider, K, Trojanowski, J, Sundman, V (1978). Screening for lignin degrading bacteria by means of 14C-labelled lignins. Archives of Microbiology, 119: 103–106
Hatakka, A (1994). Lignin-modifying enzyme from selected white-rot fungi: production and role in lignin degradation. FEMS Microbiology Review, 13: 125–135
Holt, D M (1983). Bacterial degradation of lignified wood cell walls in aerobic aquatic habitats: Decay patterns and mechanisms proposed to account for their formation. Journal of the Institute of Wood Science, 9 (5): 212–223
Holt, D M, Jones, E B (1983). Bacterial degradation of lignified wood cell walls in anaerobic aquatic habitats. Applied and Environment Microbiology, 46: 722–727
Janshekar, H, Fiechter, A (1982). On the bacterial degradation of lignin. European of Journal Applied Microbiology and Biotechnology, 14: 47–50
Jiménez, M, García-Carmona F (1999). Oxidation of the flavonol quercetin by polyphenol oxidase. Journal of Agricultural and Food Chemistry, 47: 56–60
Kaplan, D L, Hartenstein, R (1980). Decomposition of lignins by microorganisms. Soil Biology and Biochemistry, 12: 65–75
Karam, J, Nicell, J A (1997). Potential applications of enzymes in waste treatment. Journal of Chemical Technology & Biotechnology, 69:141–153
Hammel, K E, Cullen, D (2008). Role of fungal peroxidases in biological ligninolysis. Current Opinion in Plant Biology, 11: 349–355
Kern, H W (1984). Bacterial degradation of dehydropolymers of coniferyl alcohol. Archives of Microbiology, 138: 18–25
Kern, H W, Kirk, T K (1987). Influence of molecular size and ligninase pretreatment on degradation of lignins by Xanthomonas sp. Strains 99. Applied and Environment Microbiology, 53: 2242–2246
Kerr, T J, Kerr, R D, Benner, R (1983). Isolation of a bacterium capable of degrading peanut hull lignin. Applied and Environment Microbiology, 46: 1201–1206
Kim, Y S, Singh, A P, Nilsson, T (1996). Bacteria as important degraders in water-logged archaeological wood. Holzforschung, 50: 389–392
Kirk, T K, Farrell, R L (1987). Enzymatic “combustion’: the microbial degradation of lignin. Annual Review of Microbiology, 41: 465–505
Ko, C H, Chen, W L, Tsai, C H, Jane, W N, Liu, C C, Tu, J (2007). Paenibacillus campinasensis BL11: A wood material-utilizing bacterial strain isolated from black liquor. Bioresource Technology, 98: 2727–2733
Lack, A, Tommasi, I, Aresta, M, Fuchs, G (1991). Catalytic properties of phenol carboxylase. In vitro study of CO2: 4-hydroxybenzoate isotope exchange reaction. European Journal of Biochemistry, 197: 473–479
Mahadevan A (1991). Biochemical aspects of disease resistance. Part II. Post Infectional Defence Mechanisms. New Delhi: Today and Tomorrow
Matthias B, Georg F (2005). Unusual reactions involved in anaerobic metabolism of phenolic compounds. Biological chemistry, 386: 989–997
Mayer, A M, Harel, E (1979). Polyphenoloxidase in plants. Phytochemistry, 18: 193–216
McCarthy, A J (1987). Lignocellulose-degrading Actinomycetes. FEMS Microbiology Review, 46: 145–163
McMillin, D R, Eggleston M K (1997). Bioinorganic chemistry of laccase. In: Messerrschmidt A, eds. Multicopper oxidases. Singapore: World Scientific, 129–166
Minussi, R C, Pastore, G M, Durán N (2002). Potential applications of laccase in the food industry. Trends in Food Science & Technology, 13: 205–216
Modi, D R, Chandra, H, Garg, S K (1998). Decolorization of baggasebased paper mill effluent by the white-rot fungus Trametes versicolor. Bioresource Technology, 66: 79–81
Monties, B, Fukushima, K (2001). Occurrence, function, and biosynthesis of lignins. In: Steinbüchel A, Hofrichter M, eds. Biopolymers, Vol 1-Lignin, humic substances, and coal. Weinheim, Germany: Wiley-VCH, 1–64
Nagarathnamma, R, Bajpai, P, Bajpai, P K (1999). Studies on decolourization, degradation and detoxification of chlorinated lignin compounds in kraft bleaching effluents by Ceriporiopsis subvermispora. Process Biochemistry, 34: 939–948
Nie, G, Reading, S, Aust, S D (1999). Relative stability of recombinant versus native peroxidases from Phanerochaete chrysosporium. Archives of Biochemistry and Biophysics, 365(2): 328–334
Nilsson, T, Daniel, G (1992). Attempts to isolate tunnelling bacteria through physical separation from other bacteria by the use of cellophane. 23. Annual Meeting-International Research Group on Wood Preservation, Harrogate (United Kingdom), 10–15 May. Stockholm, Sweden: The International Research Group on Wood Preservation, Document No: IRG/WP/1536
Novotny, C, Svobodova, K, Erbanova, P, Cajhaml, T, Kasinath, A, Lang, E, Sasek, V (2004). Ligninolytic fungi in bioremediation: extracellular enzyme production and degradation rate. Soil Biology and Biochemistry, 36: 1545–1551
Perestelo, F, Rodriquez, A, Perez, R, Carnicero, A, Fuente, G, Falcon, M A (1996). Isolation of a bacterium capable of limited degradation of industrial and labeled natural and synthetic lignins. World Journal of Microbiology & Biotechnology, 12: 111–112
Petri, W, Andreas, K (2008). Laccase applications in the forest products industry: A review. Enzyme and Microbial Technology, 42: 293–307
Phelan, M B, Crawford, D L, Pometto, A L 3rd (1979). Isolation of lignocellulose-decomposing actinomycetes and degradation of specifically 14C-labeled lignocelluloses by six selected Streptomyces strains. Canadian Journal of Microbiology, 25: 1270–1276
Poulos, T L, Edwards, S L, Wariishi, H, Godl, M H (1993). Crystallographic refinement of lignin peroxidase at 2 Å. Journal of Biological Chemistry, 268: 4429–4440
Ralph, J, Peng, JP, Lu, FC, Hatfield, RD, Helm, RF (1999). Are lignins optically active? Journal of Agricultural and Food Chemistry, 47: 2991–2996
Ralph, K (2005). Actinomycetes and lignin degradation. Advances in Applied Microbiology, 58: 125–168
Rob, A, Ball, A S, Tuncer, M, Wilson, M T (1996). Thermostable novel non-haem extracellular glycosylated peroxidase from Thermomonospora fusca BD25. Applied Biochemistry, 24: 161–170
Robinson, L E, Crawford, D L (1978). Degradation of 14C-labeled lignins by Bacillus megaterium. FEMS Microbiology Letters, 4: 301–302
Ruttiman, G B, Vicuna, R, Sapag, C, Seelenfreund, D (1998). Biochemical and genetic studies of bacteria metabolising lignin-related compounds. Archivos de biología y medicina experimentales, 21: 247–255
Sanchez-Amat, A, Solano, F (1997). A pluripotent polyphenoloxidase from the melanogenic marine Alteromonas sp. shares catalytic capabilities of tyrosinase and laccases. Biochemical and Biophysical Research Communications, 240: 787–792
Schmeling, S, Narmandakh, A, Schmitt, O, Gad’on, N, Schühle, K, Fuchs, G (2004). Phenylphosphate synthase: a new phosphotransferase catalyzing the first step in anaerobic phenol metabolism in Thauera aromatica. Journal of Bacteriology, 186: 8044–8057
Schühle, K, Fuchs, G (2004). Phenylphosphate carboxylase: a new C-C lyase involved in anaerobic phenol metabolism in Thauera aromatica. Journal of Bacteriology, 186: 4556–4567
Shahriar, S, Ezzatollah, K, Jacqueline, K (2007). Polyphenol oxidase activity in dormant saffron (Crocus sativus L.) corm. Acta physiologiae plantarum, 29: 463–471
Singh, A P,, Nilsson, J, Daniel, G F (1990). Bacterial attack of Pinus sylvestris wood under near anaerobic conditions. Journal of the Institute of Wood Science, 12 (3): 143–157
Sjoblad, R D, Bollag, J M (1981). Oxidative coupling of aromatic pesticide intermediates by a fungal phenol oxidase. Applied and Environment Microbiology, 33: 906–910
Sjöström E (1993). Wood chemistry: fundamentals and applications. 2nd ed. San Diego, CA: Academic Press Inc.
Smith, M R, Ratledge, C (1989). Catabolism of biphenyl by Pseudomonas sp. NCIB 10643 and Nocardia sp. NCIB 10503. Applied Microbiology and Biotechnology, 30: 395–401
Srinivasan, V R, Cary, J W (1987). Biodegradation of lignin by bacteria and molecular cloning of a gene for arylether cleavage. In: Kennedy J F eds. Wood and Cellulosics. Chichester: Ellis Horwood, 267–274
Susana, R C, José, L T H (2006). Industrial and biotechnological applications of laccases: A review. Biotechnology Advances, 24: 500–513
Tabak, H H, Chambers, C W, Kabler, P W (1959). Bacterial utilization of lignans. International Journal of systematic Bacteriology, 78: 469–476
Thurston, C F (1994). The structure and function of fungal laccases. Microbiology, 140: 19–26
Trojanowski, J, Haider, K, Sundman, V (1997). Decomposition of 14Clabelled lignin and phenols by a Nocardia sp. Archives of Microbiology, 114: 149–153
Tuomela, M, Stefen, K T, Kerko, E, Hartikainen, H, Hofritcher, M, Hatakka, A (2005). Influence of Pb contamination in boreal forest soil on the growth and ligninolytic activity of litter-decomposing fungi. FEMS Microbiology Ecology, 53: 179–186
Vicuña, R (1988). Bacterial degradation of lignin. Enzyme and Microbial Technology, 10: 646–656
Watanabe, Y, Shinzato, N, Fukatsu, T (2003). Isolation of actinomycetes from termites’ guts. Bioscience Biotechnology and Biochemistry, 67: 1797–1801
Welinder K G (1992). Superfamily of plant, fungal and bacterial peroxidases. Current Opinion in Structural Biology, 2: 388–393
Winter B, Fiechter, A, Zimmerman, W (1991). Degradation of organochlorine compounds in spent sulfite bleach plant effluents by Actinomycetes. Applied and Environment Microbiology, 57: 2858–2863
Xu F (1996). Oxidation of phenols, anilines, and benzenethiols by fungal laccases: correlation between activity and redox potentials as well as halide inhibition. Biochemistry, 35: 7608–7614
Yang, J S, Ni, J R, Yuan, H L, Wang, E T (2007). Biodegradation of three different wood chips by Pseudomonas sp. PKE117. International Biodeterioration & Biodegradation, 60: 90–95
Yaropolov, A I, Skorobogat’ko, O V, Vartanov, S S, Varfolomeyer, S D (1994). Laccase: properties, catalytic mechanism, and applicability. Applied Biochemistry and Biotechnology, 49: 257–280
Zeikus, J G, Wellstein, A L, Kirk, T K (1982). Molecular basis for the biodegradative recalcitrance of lignin in anaerobic environments. FEMS Microbiology Letters, 15: 193–197
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Yuan, H. & Yang, J. Bacteria and lignin degradation. Front. Biol. China 4, 29–38 (2009). https://doi.org/10.1007/s11515-008-0097-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11515-008-0097-8