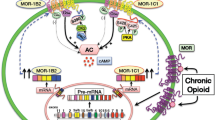
Abstract
Clinically used opioids, such as morphine, activate the mu opioid receptor (MOR) encoded by Opioid Receptor Mu 1 (OPRM1) gene. Examination of the opioid receptor genes showed that the human OPRM1 pre–mRNA undergoes extensive alternative splicing events and capable of expressing 21 isoforms. However, characterization of OPRM1 signaling is generalized, and only one isoform (MOR–1) has been extensively studied. Compounding this issue is the increasing significance of intravenous drug abuse in HIV neuropathogenesis. Here, we investigated the molecular impact of morphine and HIV–1 on regulation of OPRM1 pre–mRNA splicing in in vitro and in vivo models. Our results suggested that morphine treatment specifically induces the alternative splicing of MOR–1X isoform among the other isoforms analyzed in neuronal cells. Interestingly, alternative splicing and expression of MOR–1X isoform was also induced in postmortem brain tissues obtained from people with HIV (PWH). Additionally, treatment of control rats with morphine induced alternative splicing of MOR–1X in the brain regions involved in the reward pathways. More interestingly, HIV–1 transgenic (HIV–1Tg) rats, showed an additive induction of MOR–1X isoform with the exposure to morphine. To further assess the possible role of HIV secretory proteins in alternative splicing of OPRM1 gene, we analyzed the impact of HIV–1 Tat, gp120 and Nef proteins on alternative splicing of MOR–1X isoform. While the Tat and gp120 had no visible effects, treatment of neurons with Nef induced MOR–1X alternative splicing that was comparable to treatment with morphine. Altogether, our results suggest that HIV–1 may alter MOR isoform expression with Nef protein by amplifying the rate of MOR–1X alternative splicing induced by morphine.
Graphical abstract







Similar content being viewed by others
References
Abbadie C, Pan YX, Drake CT, Pasternak GW (2000) Comparative immunohistochemical distributions of carboxy terminus epitopes from the mu–opioid receptor splice variants MOR–1D, MOR–1 and MOR–1C in the mouse and rat CNS. Neurosci 100:141–153. https://doi.org/10.1016/S0306-4522(00)00248-7
Abbadie C, Pan YX, Pasternak GW (2004) Immunohistochemical study of the expression of EXON11–containing μ opioid receptor variants in mouse brain. Neurosci 127:419–430. https://doi.org/10.1016/j.neuroscience.2004.03.033
Abbadie C, Pasternak GW (2001) Differential in vivo internalization of MOR–1 and MOR–1C by morphine: Neuroreport 12:3069–3072. https://doi.org/10.1097/00001756-200110080-00017
Abbadie C, Pasternak GW, Aicher SA (2001) Presynaptic localization of the carboxy–terminus epitopes of the μ opioid receptor splice variants MOR–1C and MOR–1D in the superficial laminae of the rat spinal cord. Neurosci 106:833–842. https://doi.org/10.1016/S0306-4522(01)00317-7
Abbadie C, Rossi GC, Orciuolo A et al (2002) Anatomical and functional correlation of the endomorphins with mu opioid receptor splice variants: Endomorphins and mu opioid receptor splice variants. Eur J Neurosci 16:1075–1082. https://doi.org/10.1046/j.1460-9568.2002.02173.x
Avdoshina V, Biggio F, Palchik G et al (2010) Morphine induces the release of CCL5 from astrocytes: potential neuroprotective mechanism against the HIV protein gp120: morphine, astrocytes, and CCL5. Glia 58:1630–1639. https://doi.org/10.1002/glia.21035
Bardi G, Sengupta R, Khan MZ et al (2006) Human immunodeficiency virus gp120–induced apoptosis of human neuroblastoma cells in the absence of CXCR4 internalization. J Neurovirol 12:211–218. https://doi.org/10.1080/13550280600848373
Bare LA, Mansson E, Yang D (1994) Expression of two variants of the human μ opioid receptor mRNA in SK–N–SH cells and human brain. FEBS Lett 354:213–216. https://doi.org/10.1016/0014-5793(94)01129-X
Brew BJ, Rosenblum M, Cronin K, Price RW (1995) AIDS dementia complex and HIV–1 brain infection: Clinical–virological correlations. Ann Neurol 38:563–570. https://doi.org/10.1002/ana.410380404
Chang SL, Connaghan KP (2012) Behavioral and molecular evidence for a feedback interaction between morphine and HIV–1 viral proteins. J Neuroimmune Pharmacol 7:332–340. https://doi.org/10.1007/s11481-011-9324-1
Chang SL, Connaghan KP, Wei Y, Li MD (2014) NeuroHIV and use of addictive substances. Int Rev Neurobiol 118:403–440. https://doi.org/10.1016/B978-0-12-801284-0.00013-0
Chang SL, Vigorito M (2006) Role of HIV–1 infection in addictive behavior: a study of a HIV–1 transgenic rat model. Am J Infect Dis 2:98–106. https://doi.org/10.3844/ajidsp.2006.98.106
Craigie M, Cicalese S, Sariyer IK (2018) Neuroimmune regulation of JC virus by intracellular and extracellular agnoprotein. J Neuroimmune Pharmacol 13:126–142. https://doi.org/10.1007/s11481-017-9770-5
Dahal S, Chitti SVP, Nair MPN, Saxena SK (2015) Interactive effects of cocaine on HIV infection: implication in HIV–associated neurocognitive disorder and neuroAIDS. Front Microbiol. https://doi.org/10.3389/fmicb.2015.00931
Dever SM, Costin BN, Xu R et al (2014) Differential expression of the alternatively spliced OPRM1 isoform μ–opioid receptor–1K in HIV–infected individuals. AIDS 28:19–30. https://doi.org/10.1097/QAD.0000000000000113
Dever SM, Xu R, Fitting S et al (2012) Differential expression and HIV–1 regulation of μ–opioid receptor splice variants across human central nervous system cell types. J Neurovirol 18:181–190. https://doi.org/10.1007/s13365-012-0096-z
Donadoni M, Cicalese S, Sarkar DK et al (2019) Alcohol exposure alters pre–mRNA splicing of antiapoptotic Mcl–1L isoform and induces apoptosis in neural progenitors and immature neurons. Cell Death Dis 10:447. https://doi.org/10.1038/s41419-019-1673-3
Gibellini D, Alviano F, Miserocchi A et al (2011) HIV–1 and recombinant gp120 affect the survival and differentiation of human vessel wall–derived mesenchymal stem cells. Retrovirology 8:40. https://doi.org/10.1186/1742-4690-8-40
Gurwitz KT, Burman RJ, Murugan BD et al (2017) Time–dependent, HIV–Tat–induced perturbation of human neurons in vitro: towards a model for the molecular pathology of HIV–associated neurocognitive disorders. Front Mol Neurosci 10:163. https://doi.org/10.3389/fnmol.2017.00163
High KP, Brennan–Ing M, Clifford DB et al (2012) HIV and aging: state of knowledge and areas of critical need for research. a report to the NIH office of AIDS research by the HIV and aging working group. JAIDS J Acquir Immune Defic Syndr 60:S1–S18. https://doi.org/10.1097/QAI.0b013e31825a3668
Homji NF, Vigorito M, Chang SL (2012) Morphine–induced conditioned place preference and associated behavioural plasticity in HIV–1 transgenic rats. Int J Clin Exp Med 5:105–123
Hui J (2009) Regulation of mammalian pre-mRNA splicing. Sci China Ser C 52:253–260. https://doi.org/10.1007/s11427-009-0037-0
Jana A, Pahan K (2004) Human immunodeficiency virus type 1 gp120 induces apoptosis in human primary neurons through redox–regulated activation of neutral sphingomyelinase. J Neurosci 24:9531–9540. https://doi.org/10.1523/JNEUROSCI.3085-04.2004
Kennedy CA, Zerbo E (2014) HIV–related neurocognitive disorders and drugs of abuse: mired in confound, surrounded by risk. Curr Addict Rep 1:229–236. https://doi.org/10.1007/s40429-014-0028-5
Keren H, Lev–Maor G, Ast G (2010) Alternative splicing and evolution: diversification, exon definition and function. Nat Rev Genet 11:345–355. https://doi.org/10.1038/nrg2776
Khan MB, Lang MJ, Huang M–B et al (2016) Nef exosomes isolated from the plasma of individuals with HIV–associated dementia (HAD) can induce Aβ(1–42) secretion in SH–SY5Y neural cells. J Neurovirol 22:179–190. https://doi.org/10.1007/s13365-015-0383-6
Kim E, Goren A, Ast G (2008) Alternative splicing: current perspectives. BioEssays 30:38–47. https://doi.org/10.1002/bies.20692
Koch T, Schulz S, Pfeiffer M et al (2001) C–terminal splice variants of the mouse µ–opioid receptor differ in morphine–induced internalization and receptor resensitization. J Biol Chem 276:31408–31414. https://doi.org/10.1074/jbc.M100305200
Koch T, Schulz S, Schro¨der H et al (1998) Carboxyl–terminal splicing of the rat μ opioid receptor modulates agonist–mediated internalization and receptor resensitization. J Biol Chem 273:13652–13657. https://doi.org/10.1074/jbc.273.22.13652
Kvam T–M, BaarRakvåg CTT et al (2004) Genetic analysis of the murine µ opioid receptor: increased complexity of Oprm gene splicing. J Mol Med 82:250–255. https://doi.org/10.1007/s00109-003-0514-z
McNamara RP, Costantini LM, Myers TA et al (2018) Nef secretion into extracellular vesicles or exosomes is conserved across human and simian immunodeficiency viruses. mBio 9. https://doi.org/10.1128/mBio.02344-17
Mizoguchi H, Wu H–E, Narita M et al (2003) Lack of μ–opioid receptor–mediated g–protein activation in the spinal cord of mice lacking exon 1 or exons 2 and 3 of the MOR–1 Gene. J Pharmacol Sci 93:423–429. https://doi.org/10.1254/jphs.93.423
Mohseni Ahooyi T, Shekarabi M, Decoppet EA et al (2018) Network analysis of hippocampal neurons by microelectrode array in the presence of HIV–1 Tat and cocaine. J Cell Physiol 233:9299–9311. https://doi.org/10.1002/jcp.26322
Molitor F, Truax SR, Ruiz JD, Sun RK (1998) Association of methamphetamine use during sex with risky sexual behaviors and HIV infection among non–injection drug users. West J Med 168:93–97
Natarajaseenivasan K, Cotto B, Shanmughapriya S et al (2018) Astrocytic metabolic switch is a novel etiology for Cocaine and HIV–1 Tat–mediated neurotoxicity. Cell Death Dis 9:415. https://doi.org/10.1038/s41419-018-0422-3
Nath A, Hauser KF, Wojna V et al (2002) Molecular basis for interactions of HIV and drugs of abuse: JAIDS. J Acquir Immune Defic Syndr 31:S62–S69. https://doi.org/10.1097/00126334-200210012-00006
Oldfield S, Braksator E, Rodriguez–Martin I et al (2008) C–terminal splice variants of the μ–opioid receptor: existence, distribution and functional characteristics. J Neurochem 104:937–945. https://doi.org/10.1111/j.1471-4159.2007.05057.x
Pan L, Xu J, Yu R et al (2005) Identification and characterization of six new alternatively spliced variants of the human μ opioid receptor gene, Oprm. Neuroscience 133:209–220. https://doi.org/10.1016/j.neuroscience.2004.12.033
Pan YX, Xu J, Mahurter L et al (2003) Identification and characterization of two new human mu opioid receptor splice variants, hMOR–1O and hMOR–1X. Biochem Biophys Res Commun 301:1057–1061. https://doi.org/10.1016/S0006-291X(03)00089-5
Pasternak DA, Pan L, Xu J et al (2004) Identification of three new alternatively spliced variants of the rat mu opioid receptor gene: dissociation of affinity and efficacy. J Neurochem 91:881–890. https://doi.org/10.1111/j.1471-4159.2004.02767.x
Pasternak GW (2010) Molecular insights into μ opioid pharmacology: from the clinic to the bench. Clin J Pain 26:S3–S9. https://doi.org/10.1097/AJP.0b013e3181c49d2e
Ranki A, Nyberg M, Ovod V et al (1995) Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS 9:1001–1008. https://doi.org/10.1097/00002030-199509000-00004
Ravindranathan A, Joslyn G, Robertson M et al (2009) Functional characterization of human variants of the mu–opioid receptor gene. Proc Natl Acad Sci 106:10811–10816. https://doi.org/10.1073/pnas.0904509106
Raymond AD, Campbell–Sims TC, Khan M et al (2011) HIV Type 1 Nef is released from infected cells in CD45(+) microvesicles and is present in the plasma of HIV–infected individuals. AIDS Res Hum Retroviruses 27:167–178. https://doi.org/10.1089/aid.2009.0170
Raymond AD, Diaz P, Chevelon S et al (2016) Microglia–derived HIV Nef+ exosome impairment of the blood–brain barrier is treatable by nanomedicine–based delivery of Nef peptides. J Neurovirol 22:129–139. https://doi.org/10.1007/s13365-015-0397-0
Reddy PVB, Pilakka–Kanthikeel S, Saxena SK et al (2012) Interactive effects of morphine on HIV infection: role in HIV–associated neurocognitive disorder. AIDS Res Treat 2012:1–10. https://doi.org/10.1155/2012/953678
Regan PM, Sariyer IK, Langford TD et al (2016) Morphine–induced MOR–1X and ASF/SF2 expressions are independent of transcriptional regulation: implications for MOR–1X signaling: MORPHINE–INDUCED MOR–1X AND ASF/SF2 EXPRESSIONS. J Cell Physiol 231:1542–1553. https://doi.org/10.1002/jcp.25246
Saha RN, Pahan K (2003) Tumor necrosis factor–α at the crossroads of neuronal life and death during HIV–associated dementia: TNF–α and HIV–associated dementia. J Neurochem 86:1057–1071. https://doi.org/10.1046/j.1471-4159.2003.01942.x
Saribas AS, Cicalese S, Ahooyi TM et al (2017) HIV–1 Nef is released in extracellular vesicles derived from astrocytes: evidence for Nef–mediated neurotoxicity. Cell Death Dis 8:e2542. https://doi.org/10.1038/cddis.2016.467
Saylor D, Dickens AM, Sacktor N et al (2016) HIV–associated neurocognitive disorder–pathogenesis and prospects for treatment. Nat Rev Neurol 12:234–248. https://doi.org/10.1038/nrneurol.2016.27
Shabalina SA, Zaykin DV, Gris P et al (2009) Expansion of the human μ–opioid receptor gene architecture: novel functional variants. Hum Mol Genet 18:1037–1051. https://doi.org/10.1093/hmg/ddn439
Ständer S, Gunzer M, Metze D et al (2002) Localization of mu–opioid receptor 1A on sensory nerve fibers in human skin. Regul Pept 110:75–83. https://doi.org/10.1016/s0167-0115(02)00159-3
Tanowitz M, Hislop JN, von Zastrow M (2008) Alternative splicing determines the post–endocytic sorting fate of G–protein–coupled Receptors. J Biol Chem 283:35614–35621. https://doi.org/10.1074/jbc.M806588200
Tarn W–Y (2007) Cellular signals modulate alternative splicing. J Biomed Sci 14:517–522. https://doi.org/10.1007/s11373-007-9161-7
Tiwari S, Nair MP, Saxena SK (2013) Latest trends in drugs of abuse – HIV infection and neuroAIDS. Future Virol 8:121–127. https://doi.org/10.2217/fvl.12.134
Vakili S, Ahooyi TM, Yarandi SS et al (2020) Molecular and cellular impact of inflammatory extracellular vesicles (EVs) derived from M1 and M2 macrophages on neural action potentials. Brain Sci 10:424. https://doi.org/10.3390/brainsci10070424
Vigorito M, Connaghan KP, Chang SL (2015) The HIV–1 transgenic rat model of neuroHIV. Brain Behav Immun 48:336–349. https://doi.org/10.1016/j.bbi.2015.02.020
World Drug Report (2020) (United nations publication, Sales No. E.20.XI.6)
Yarandi SS, Duggan MR, Sariyer IK (2021) Emerging role of nef in the development of HIV associated neurological disorders. J Neuroimmune Pharmacol 16:238–250. https://doi.org/10.1007/s11481-020-09964-1
Zhang Y, Pan Y–X, Kolesnikov Y, Pasternak GW (2006) Immunohistochemical labeling of the mu opioid receptor carboxy terminal splice variant mMOR–1B4 in the mouse central nervous system. Brain Res 1099:33–43. https://doi.org/10.1016/j.brainres.2006.04.133
Acknowledgements
Authors wants to thank to present and past members of the Neuroscience Department and Center for Neurovirology at Lewis Katz School of Medicine (LKSOM) for sharing of ideas and reagents.
Funding
This work was supported, in part, by grants (DA07058, DA043448, AA025398) awarded by the NIH to IKS, SLC, and THB. SSY was supported by Interdisciplinary and Translational Research Training in NeuroAIDS (T32MH079785). The study utilized services offered by core facilities of the Comprehensive NeuroAIDS Center (CNAC NIMH Grant Number P30MH092177) at LKSOM at Temple University. This publication was made possible from NIH funding through the NIMH and NINDS Institutes by the following grants: Texas NeuroAIDS Research Center: U24MH100930, California NeuroAIDS Tissue Network: U24MH100928, National Neurological AIDS Bank: U24MH100929, Manhattan HIV Brain Bank: U24MH100931, Data Coordinating Center: U24MH100925. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NNTC or NIH.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: IKS and SLC. Performed the experiments: MD, WH, SSY. Analyzed the data: MD, WH, IKS and SLC. Contributed reagents/materials/analysis tools: IKS, THB, SLC. Wrote the paper: IKS, SLC, and MD.
Corresponding authors
Ethics declarations
Ethics Approval
Human postmortem brain samples were obtained and used in accordance with Temple University Human Subjects Protections, with the approval of the institutional review board.
The experimental protocol using HIV–1Tg and F344 rats was approved by the Institutional Animal Care and Use Committee (IACUC) at Seton Hall University, South Orange, NJ.
Conflicts of Interest/Competing Interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Donadoni, M., Huang, W., Yarandi, S.S. et al. Modulation of OPRM1 Alternative Splicing by Morphine and HIV–1 Nef. J Neuroimmune Pharmacol 17, 277–288 (2022). https://doi.org/10.1007/s11481-021-10009-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-021-10009-4