Abstract
Autoimmune neurological disorders are commonly treated with immunosuppressive therapy. In patients with refractory conditions, standard immunosuppression is often insufficient for complete recovery or to prevent relapses. These patients rely on other treatments to manage their disease. While treatment of refractory cases differs between diseases, intravenous immunoglobulin, plasma exchange (PLEX), and immune-modulating treatments are commonly used. In this review, we focus on five autoimmune neurological disorders that were the themes of the 2018 Midlands Neurological Society meeting on PLEX in refractory neurology: Autoimmune Encephalitis (AE), Multiple Sclerosis (MS), Neuromyelitis Optica Spectrum disorders (NMOSD), Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP) and Myasthenia Gravis (MG). The diagnosis of inflammatory neuropathies is often challenging, and while PLEX can be very effective in refractory autoimmune diseases, its ineffectiveness can be confounded by misdiagnosis. One example is POEMS syndrome (characterized by Polyneuropathy Organomegaly, Endocrinopathy, Myeloma protein, Skin changes), which is often wrongly diagnosed as CIDP; and while CIDP responds well to PLEX, POEMS does not. Accurate diagnosis is therefore essential. Success rates can also differ within ‘one’ disease: e.g. response rates to PLEX are considerably higher in refractory relapsing remitting MS compared to primary or secondary progressive MS. When sufficient efforts are made to correctly pinpoint the diagnosis along with the type and subtype of refractory autoimmune disease, PLEX and other immunotherapies can play a valuable role in the patient management.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are numerous autoimmune disorders involving the central nervous system (CNS) and the peripheral nervous system (PNS). They are characterized by abnormal immune responses against antigens expressed within the nervous system. However, the presentation and severity of these diseases can vary greatly (Rubin et al. 2018).
The standard of care for autoimmune neurological disorders in relapse is corticosteroids and immunosuppressive therapy (e.g. azathioprine, mycophenolate) to prevent recurrence of the symptoms. However, a proportion of patients are refractory (i.e. they do not respond to these treatments). Intravenous immunoglobulins (IVIg) and plasma exchange (PLEX) are also widely used to treat neurological disorders, with various degrees of efficacy observed for different disorders (Lehmann and Hartung 2011; Linker and Gold 2008). More recently, immunoadsorption has also emerged as a potential alternative to PLEX for the treatment of neurological disorders. While PLEX consists of fluid replacement with a blood solution such as fresh frozen plasma or albumin, immunoadsorption is a blood purification process through which humoral factors (i.e. disease-specific autoantibodies) can be removed from separated plasma by using a high-affinity adsorbent (Oji and Nomura 2017). The Midlands Neurological Society meeting on PLEX in refractory neurology highlighted five different autoimmune neurological disorders and the available treatment options, in particular the role of PLEX for patients experiencing refractory conditions of the disease. PLEX procedure considerations in these neurological diseases are provided in Table 1.
In the current review, we focus on the five autoimmune neurological disorders that were presented at the meeting of 2018: Autoimmune Encephalitis (AE), Multiple Sclerosis (MS), Neuromyelitis Optica Spectrum Disorder (NMOSD), Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP) and Myasthenia Gravis (MG). Most of the authors are practicing neuroimmunologists with expertise in specific diseases and aim to provide an overview of the literature and in particular their own perspectives.
Autoimmune Encephalitis
AE is generally characterized by impaired memory and cognition, often with seizures and/or a movement disorder and sometimes with reduced consciousness or coma, but presentations vary widely across various sub-types (Broadley et al. 2019; Ramanathan et al. 2019). As its laboratory and imaging profiles are frequently normal, AE can be difficult to diagnose.
AE has an incidence of around 0.8/100,000 person-years (Dubey et al. 2018). A small but significant proportion of AE patients is refractory to first- and second-line treatment (Shin et al. 2018), and almost all patients have residual cognitive deficits.
First-line treatment of AE consists of steroids (e.g. methylprednisolone), IVIg and PLEX or immunoadsorption, with corticosteroids being the first choice in most cases. Second-line immunotherapy often consists of rituximab or cyclophosphamide (Shin et al. 2018; Thompson et al. 2018; Titulaer et al. 2013).
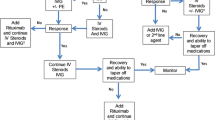
While corticosteroids are generally the first choice, treatment with steroids alone is often insufficient to achieve adequate clinical improvements. In these cases, it may be useful to combine steroid treatment with PLEX or IVIg administration to obtain a synergistic effect (Shin et al. 2018). It has been shown that simultaneous PLEX and intravenous methylprednisolone (IVMP) treatment followed transitioning to an IVIg regimen results in improved outcomes on the short term (1–2 months) compared to simultaneous IVIg and IVMP treatment without PLEX (Zhang et al. 2021). PLEX is also thought to positively impact AE by stimulating the proliferation of autoantigen-specific B cells, thereby increasing their susceptibility to immunosuppressants and chemotherapeutic agents (Reeves and Winters 2014; Shin et al. 2018). Response rates of around 65% have been reported for PLEX in refractory AE in a systematic literature review of 120 patients (Suppiej et al. 2016), as well as a retrospective review (DeSena et al. 2015) and a pilot study (Heine et al. 2016) (Table 2). In the latter, the percentage of patients experiencing improvements after PLEX was 83% among those with neuronal cell surface antibodies and 66.7 % among those with antibodies against intracellular-synaptic sites, while none of the patients with intracellular antigens experienced improvements after PLEX (Heine et al. 2016). A recent retrospective study found that 75% of patients with AE respond to PLEX, which also had a favourable safety profile (Moser et al. 2019). Several other studies have also confirmed the efficacy of PLEX in AE (Fassbender et al. 2017). PLEX also seems to be effective in children with AE (Prytuła et al. 2015; Wright et al. 2015).
Multiple Sclerosis
MS is a chronic inflammatory, demyelinating and degenerative disease of the CNS. MS, the most common demyelinating disease, has a prevalence ranging from 2/100,000 population in Eastern Asia and sub-Saharan Africa to more than 100/100,000 inhabitants in North America and Europe (Leray et al. 2016). MS affects >120,000 people in the UK (Mackenzie et al. 2014). The symptomatology of MS varies widely depending on the degree of damage and site of inflammatory, demyelinating and degenerative changes.
The most common type of MS is relapsing remitting MS (RRMS), characterized by relapses and remissions. Incomplete resolution of relapses is associated with accumulation of neurological disability (Lublin et al. 2003). The natural history of RRMS is one of progression, i.e. development of secondary progressive MS (SPMS). If untreated (disease-modifying drugs), the majority of people with RRMS will develop significant neurological disability within 10 years of onset, and 50% will require wheelchair assistance within 20 years. A minority of patients develop primary progressive MS (PPMS), characterized by a gradual progression of neurological disability (Fitzner and Simons 2010).
The standard treatment of MS relapses consists of immunosuppression with corticosteroids, but about 5 to 20% of patients with MS relapses may fail to respond to steroids (Brusaferri and Candelise 2000). The aim of a relapse treatment is to accelerate functional recovery after inflammatory demyelination, alleviate the severity of the relapse and decrease the chance of persistent neurological deficit. Corticosteroids are strong anti-inflammatory agents that exert their actions through various mechanisms including activation of the glucocorticoid receptor and disruption of the mitochondrial membrane potential resulting in apoptosis of T cells, decreasing migration of inflammatory cells into the CNS through decrease expression of adhesion molecules VLA-4 and LFA-1 (Elovaara et al. 1998) and a decrease in intrathecal synthesis of IgG (Sellebjerg et al. 2000).
Patients with steroid-refractory relapses can benefit from PLEX, with reported response rates of 40 to 90% (Stork et al. 2018). The American Academy of Neurology (AAN) (Cortese et al. 2011) guidelines state that PLEX should be considered for the adjunctive treatment of exacerbations in relapsing forms of MS (Level B). PLEX may be considered in the treatment of fulminant CNS demyelinating diseases that fail to respond to high-dose corticosteroid treatment (Level C; the available evidence did not allow to make a recommendation for MS separately). PLEX should not be offered for PPMS or SPMS (Level A) (Cortese et al. 2011). The benefit of PLEX in patients with steroid-refractory relapses is further illustrated by recent studies (Table 2). A Portuguese retrospective cohort study evaluated 46 patients with severe acute relapses of MS, the majority of whom were refractory to corticosteroids. Corticosteroids were used in 94% of cases, without any immediate benefit in 37% and only mild disability recovery in the remaining cases. PLEX was initiated at 33 (±24) days after relapse onset, and 80% of the patients showed recovery after a mean of 7.4 PLEX sessions, with 41% reaching complete recovery (assessed using the Expanded Disability Status Scale [EDSS]) and 39% partial recovery (Correia et al. 2018).
Another study indicated that the response of steroid-refractive relapses to apheresis may depend on the patient’s histopathological type of disease. The study compared response to apheresis in patients with three histopathologically classified immunological patterns: T cell- and macrophage-associated demyelination (pattern 1), T cell- and macrophage-associated demyelination with immunoglobulin and complement deposits (pattern 2), and oligodendrocyte degeneration (pattern 3). Neurological recovery was observed in five of the 16 patients with pattern 1 disease (31%) and 22 of the 40 patients with pattern 2 disease (55%), but none of the 13 patients with pattern 3 disease exhibited improvement (pattern 2 vs 3 P < .001). When measured by EDSS, the corresponding response rates were 25%, 40% and 0%. Radiological improvements were found in 4 (25%), 22 (56%), and 1 (11%) of patients with patterns 1, 2, and 3, respectively (Stork et al. 2018).
A recent retrospective two-center study compared two types of apheresis: PLEX and immunoadsorption (Lipphardt et al. 2019). Immunoadsorption provides a more selective approach allowing elimination of certain proteins such as antibodies while sparing other plasma proteins (Schroder et al. 2009). The authors concluded that immunoadsorption is equally effective and safe as PLEX in steroid-resistant MS relapses. The highest response rate (74%) to apheresis treatment was observed in patients with RRMS or clinical isolated syndrome (CIS). Interestingly, although the response rate was lower in patients with PPMS/SPMS, 50% of the 22 patients benefited from apheresis (Lipphardt et al. 2019). This observation implies that apheresis could be considered as escalation therapy in progressive MS as well (Lipphardt et al. 2019).
Neuromyelitis Optica Spectrum Disorder
NMOSD is a CNS disorder that predominantly affects the optic nerves (optic neuritis) and the spinal cord (myelitis), and is mediated by aquaporin-4 immunoglobulin G (IgG) antibodies (AQP4-IgG) in most cases. A proportion of patients with similar presentations have myelin oligodendrocyte glycoprotein IgG antibodies (MOG-IgG), which are also likely pathogenic. NMOSD incidence ranges from 0.053 to 0.4/100,000 person-years (Etemadifar et al. 2015). Currently, both the acute relapses in MOG-IgG disease and AQP4-IgG NMOSD are treated similarly. For the purposes of this review, refractory NMOSD is defined as incomplete or slow recovery from an acute attack, despite corticosteroid treatment. For recurrent attacks, refractory disease is defined as relapses despite treatment with corticosteroids, azathioprine (or mycophenolate) and rituximab.
PLEX in Acute Attacks
PLEX is recommended in the AAN (2011) guidelines for the treatment of fulminant CNS demyelinating diseases that fail to respond to high-dose corticosteroid treatment. These CNS demyelinating diseases include MS, acute disseminated encephalomyelitis (ADEM), NMOSD and transverse myelitis; the study results did not allow to determine if effectiveness of PLEX varies between the different diseases (Cortese et al. 2011). PLEX is the established second-line therapy in case of steroid resistance in NMOSD in the German and United Kingdom guidelines on treatment of NMOSD (Palace et al. 2012; Trebst et al. 2014).
Reports on the benefit of PLEX are summarized in Table 2. In acute attacks, a response to PLEX has been observed in 35–65% of patients with NMOSD. While PLEX is most effective in the weeks after an acute attack, response has been seen even up to 3 months after onset of the relapse (Abboud et al. 2016; Bonnan et al. 2018). A large retrospective review of 871 relapses treated across Germany supports the escalation from steroids to PLEX, finding that it improved outcomes, particularly in transverse myelitis relapses where PLEX may even be superior to steroids (Kleiter et al. 2016).
A retrospective cohort study focusing specifically on NMO reported that adding PLEX to high-dose IVMP improved the outcome at discharge and on follow-up compared to IVMP alone. Among IVMP + PLEX patients, 65% achieved an EDSS equal or below their baseline at approximately one year follow-up, compared to 35% of the IVMP-only patients (Abboud et al. 2016).
A recent retrospective study comparing PLEX and immunoadsorption, described above for MS, also evaluated 12 patients with NMO. A positive response rate of 67% (8/12 patients) was observed after apheresis treatment. Only two of the 12 patients were treated with immunoadsorption; one of them showed a moderate response while the other improved rapidly after immunoadsorption but lacked sufficient follow-up data (Lipphardt et al. 2019).
When PLEX is ineffective for acute attacks, additional measures that may offer some benefit include IVIg, cyclophosphamide, complement inhibitors (C1 esterase inhibitor [cinryze] and C5 inhibitor [eculizumab]) neutrophil inhibitors (neutrophil elastase inhibitor [sivelestat] and neutrophil migration inhibitor [colchicine]) and eosinophil inhibitors (antihistamines [cetirizine, ketotifen]).
PLEX for Relapse Prevention
For relapse prevention, until recently, PLEX was used when conventional drugs were exhausted (e.g. azathioprine, mycophenolate, rituximab). The typical regime is two to five exchanges given every four to eight weeks based on response, and used in conjunction with a more conventional agent (e.g. azathioprine + steroids).
A case series of four patients with NMO who underwent PLEX following intensive intravenous corticosteroid therapy reported that all patients showed definite functional improvement after one or several courses of PLEX; two of these patients continued to be treated with intermittent PLEX because of disease refractory to oral agents (Miyamoto and Kusunoki 2009). In a case series of seven patients with NMO refractory to high-dose corticosteroids, an improvement was observed for all patients. In five patients who interrupted their maintenance PLEX therapy, a clinical worsening was observed (Khatri et al. 2012). This finding is currently being further investigated in about 14 patients participating in the “Maintenance Plasma Exchange for Neuromyelitis Optica (MultiPLEX)” prospective observational study (ClinicalTrials.gov: NCT01500681).
It is likely that the three newly approved relapse prevention drugs for NMOSD (eculizumab, satralizumab and inebilizumab) will reduce the need for PLEX.
Chronic Inflammatory Demyelinating Polyradiculoneuropathy
CIDP is characterized by motor deficits (i.e. numbness and paresthesia followed by weakness), with symptoms gradually worsening over time (Dyck and Tracy 2018). Reported CIDP prevalence per 100,000 population is: 1.61 in Japan (Iijima et al. 2008), 1.90 in New South Wales, Australia (McLeod et al. 1999), 2.84 South East England (Mahdi-Rogers and Hughes 2014), 7.70 Vest-Agder, Norway (Mygland and Monstad 2001).
Standard treatment of CIDP consists of steroids, IVIg, or PLEX. An overview of studies evaluating PLEX to treat CIDP is provided in Table 2.
In a study on patients with chronic inflammatory neuropathies in southeast England, treatment response in patients with CIDP was observed for 68% of the patients treated with corticosteroids, for 63% of those treated with IVIg and for 42% of those treated with PLEX (Mahdi-Rogers and Hughes 2014). Another study reported a response to steroids of 64%, while 78% responded to IVIg and 56% to PLEX. This study noted significant adverse effects of steroid treatment (diabetes, high blood pressure, duodenal ulcer, osteoporosis, psychosis and obesity) and PLEX (difficult access to veins, and deficit of blood coagulation factors) (Cocito et al. 2010).
A 2015 Cochrane systematic literature review (Mehndiratta et al. 2015) concluded that PLEX provides significant short-term improvement in disability, clinical impairment and motor nerve conduction velocity in CIDP, but rapid deterioration may occur afterwards, as based on moderate- to high-quality evidence from two small trials. Adverse events were not uncommon and related to difficulty with venous access, use of citrate and hemodynamic changes (Mehndiratta et al. 2015). AAN (2011) guidelines state that PLEX should be offered as a short-term treatment for patients with CIDP (Level A), while the role of PLEX in the long-term management of CIDP remains to be clarified (Cortese et al. 2011).
A number of small-scale studies indicate that immunoadsorption could constitute a promising and well-tolerated therapeutic alternative for CIDP patients refractory to first-line treatment options, both for short-term and long-term treatment (Dorst et al. 2018; Galldiks et al. 2011). A comparison of tryptophan immunoadsorption with PLEX indicated that immunoadsorption is at least equally effective and safe as PLEX in CIDP patients, with 67% of patients showing clinical improvement after immunoadsorption compared to 44% after PLEX (Lieker et al. 2017).
Importantly, a wrong diagnosis could be the reason for a patient presenting with CIDP that appears resistant to treatment (steroids, IVIg and PLEX). A retrospective study of 59 patients referred with a diagnosis of CIDP found that almost half of these patients (47%) failed to meet minimal CIDP diagnostic requirements (Allen and Lewis 2015). One of the diseases that is often mistaken for CIDP is POEMS, a paraneoplastic syndrome caused by an underlying plasma cell neoplasm. The syndrome is defined by the presence of a peripheral neuropathy (P), a monoclonal plasma cell disorder (M), and other paraneoplastic features, the most common of which include organomegaly (O), endocrinopathy (E), skin changes (S), but they may also have papilledema, edema, effusions, ascites and thrombocytosis. The characteristics of the neuropathy in this syndrome are similar to CIDP, hence explaining the difficulties in diagnosis (Dispenzieri 2005, 2007).
Myasthenia Gravis
MG is an autoimmune disease characterized by weakness of skeletal muscles and, in over 80% of patients, the presence of autoantibodies to the acetylcholine receptor. Generally, MG occurs more frequently in younger women and older men (Alshekhlee et al. 2009). Estimates of incidence rates vary from three to 30 cases/100,000 person-years (McGrogan et al. 2010).
Treatment of MG consists of cholinesterase inhibitors, steroids and steroid-sparing drugs (e.g. azathioprine, ciclosporin, methotrexate, cyclophosphamide, mycophenolate and rituximab). The AAN (2011) guidelines state that, because of the lack of randomized controlled studies with masked outcomes, there is insufficient evidence to support or refute the efficacy of PLEX in the treatment of myasthenic crisis or MG prethymectomy (Level U for both indications) (Cortese et al. 2011). Similarly, a 2002 Cochrane systematic literature review found no adequate randomized controlled trials. However, the authors did point out that many case series report short-term benefit from PLEX in MG, especially in myasthenic crisis (Gajdos et al. 2002). In the International Consensus Guidance for Management of Myasthenia Gravis, PLEX or IVIg is recommended in combination with high-dose steroids for patients who develop overt MG secondary to immune checkpoint inhibitor treatment (Narayanaswami et al. 2021). The relative rarity of the condition and lack of ambiguity among clinicians (most of whom find PLEX and IVIg to be effective) may be the reason why randomized controlled trials have not been conducted. Reports on the positive impact of PLEX on therapy-refractive MG go back as far as 1977 (Dau et al. 1977) (Table 2).
In current practice, PLEX and IVIg are commonly used to manage myasthenic exacerbations and crises due to their rapid onset of action (Bershad et al. 2008; Sanders et al. 2016). In a comparative study, the two therapies had comparable efficacy and were equally tolerated in adult patients with moderate to severe MG (Barth et al. 2011). IVIg and PLEX are generally not ideal for long-term maintenance therapy due to the short duration of benefits and to the associated side effects (Bershad et al. 2008; Sanders et al. 2016).
About 10–15% of patients with MG are considered to be refractory to standard treatments (Hoffmann and Meisel 2018; Silvestri and Wolfe 2014). Various definitions are being used for refractory MG. Therapy-refractory MG can be defined as chronic courses with moderate to severe symptoms or functional impairment, and is further characterized by ineffective (expanded) standard therapy, repeated myasthenic crises or severe exacerbation, and repeated need for therapy escalation with IVIg, PLEX or immunoadsorption; or in which standard therapy has unacceptable side effects, or there is contraindication for standard therapies due to comorbidities (Hoffmann and Meisel 2018). The Myasthenia Gravis Foundation of America defines refractory MG as “Post-intervention status is unchanged or worse after corticosteroids and at least two other immunosuppressive agents, used in adequate doses for an adequate duration, with persistent symptoms or side effects that limit functioning, as defined by patient and physician” (Sanders et al. 2016).
Escalation strategies recommended for treatment of refractory MG consist of regular IVIg or PLEX, cyclophosphamide and rituximab; other recommendations also include eculizumab, stem cell transplant and newer immunotherapies under trial (Hoffmann and Meisel 2018). As an alternative for PLEX in long-term treatment, immunoadsorption can also be very suitable, as its mechanism of action is more selective (Mantegazza and Antozzi 2018).
Discussion
In this paper, we have presented an overview of five neurological diseases and the treatment options for patients who are refractory to standard immunosuppression, with a focus on the role of PLEX. Author’s practice is summarized in Table 3.
PLEX is a standard first-line therapy in some autoimmune neurological conditions. Following failure or relapse after corticosteroid treatment, PLEX has also shown high response rates in multiple settings. However, the diagnosis of inflammatory neuropathies is often challenging, and ineffectiveness of PLEX can be confounded by misdiagnosis. For instance, POEMS is often wrongly diagnosed as CIDP (Dispenzieri 2005, 2007), and while PLEX is commonly used and effective in the treatment of CIDP, patients with POEMS do not respond well to PLEX (Codron et al. 2017; Dispenzieri 2007).
Even within one disease, not all refractory patients respond to PLEX. This difference may be due to varying immunological aspects in different patients. Additionally, correlations have been uncovered between efficacy of PLEX and different types or patterns within one disease. For example in refractory MS, patients with RRMS respond better to PLEX than patients with SPMS or PPMS (Cortese et al. 2011; Lipphardt et al. 2019).
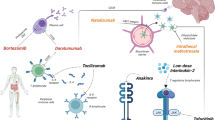
The immunomodulatory aspects of PLEX are not yet fully understood. The most logical method of action in autoimmune diseases is the removal of pathological antibodies. Additionally, PLEX can improve response to other therapies; for instance, by stimulating proliferation of B cells, thereby sensitizing them to immunosuppressants (Reeves and Winters 2014; Shin et al. 2018). Indeed, it has been shown that autoantigen-specific B cells can be observed at high frequencies in the blood of patients with several diseases mediated by autoantibodies, including AQP4, leucine-rich, glioma inactivated 1(LGI1), N-methyl D-aspartate receptor (NMDAR) and MOG antibodies (Makuch et al. 2018; Ramberger et al. 2020; Wilson et al. 2018; Winklmeier et al. 2019). Other potential ways in which PLEX can positively impact a disease is through a correction of altered T helper cell (Th) type ratio favoring Th1, changing lymphocyte numbers (more T cells and fewer B cells), increasing T-regulatory cells and T-suppressor activity, removal of immune complexes that enhances macrophage/monocyte function, removal of cytokines, and finally replacement of missing plasma components (Fig. 1, adapted from http://www.pulseline.com.au/community/therapeutic-plasma-exchange) (Reeves and Winters 2014). In autoantibody-mediated diseases, it is likely that these changes have an end-effector function on the autoantigen-specific B cells.
IVIg presents another therapy commonly used to treat refractory neuropathies. It is thought that IVIg and PLEX are similar from a clinical perspective, as for example shown for refractory MG (Barth et al. 2011). However, for certain subsets of patients that are discussed here, PLEX seems to be more beneficial. For example, as concluded in the International Consensus Guidance on the Management of Myasthenia Gravis, PLEX may be more effective than IVIg in MuSK-positive MG and the efficacy of IVIg is less certain in milder MG or ocular MG (Prytuła et al. 2015; Sanders et al. 2016). While anti-AChR antibodies, the most common autoantibodies in MG patients, are predominantly IgG1 and IgG3, anti-MuSK antibodies are predominantly IgG4 (Rivner et al. 2018), which seems to be associated with poor response to IVIg both in MG and CIDP (Wright et al. 2015). IgG4-isotype antibodies do not bind to Ig Fc-receptors and do not activate complement, which may explain the poor response to IVIg when these are involved in the pathogenic mechanism of action (Moser et al. 2019).
One of the perceived advantages of IVIg compared to PLEX is its ease of use, especially in the situation when PLEX is administered via a central line. However, the usage of centrifugal PLEX machines as opposed to the filtration-based ones makes peripheral access possible in over 70% of all cases, and can be as high as over 90% for neurological patients (Bonnan et al. 2018; Fassbender et al. 2017). Peripheral access is minimizing the potential vascular-access-related complications of PLEX and is drastically reducing the rate of catheter-induced infection (Codron et al. 2017; Elovaara and Hietaharju 2010). Peripheral access contributes to the safety and tolerability of PLEX and enables outpatient scenario.
As indicated earlier, delay in PLEX initiation is associated with worsened clinical outcomes. Therefore, having proper planning and priority access for the emergency patients facilitates this treatment option. In the current situation of IVIg shortage, provision of PLEX can help to fill the gap for those patient categories where these two therapies are equally effective (Elovaara and Hietaharju 2010; Kozanoglu et al. 2015; santé 2020).
Finally, both of these treatments have their counter-indications, for example hemodynamic instability, sepsis and hypersensitivity to albumin for PLEX, and renal failure, hypercoagulable states and hypersensitivity to immunoglobulin for IVIg.
Further research will be needed to fully understand the biological mechanisms of PLEX in refractory neurological diseases, to better understand differences in response between the different diseases and subtypes, and to ascertain its place in therapy. Randomized clinical trials provide the highest level of evidence to answer this question. However, such studies are difficult to set up as refractory disease represents only a small subset of already rare conditions.
Conclusion
The management of the refractory neurological diseases can be challenging. We provide here a fair and balanced review with a focus on the role of PLEX therapy. Further research on the mechanisms of action will help to stratify the patients into groups more likely to benefit from the therapies discussed. Finally, peripheral access and seamless provision are important elements of managing refractory neurology patients with PLEX.
References
Abboud H, Petrak A, Mealy M, Sasidharan S, Siddique L, Levy M (2016) Treatment of acute relapses in neuromyelitis optica: Steroids alone versus steroids plus plasma exchange. Mult Scler 22:185–192. https://doi.org/10.1177/1352458515581438
Allen JA, Lewis RA (2015) CIDP diagnostic pitfalls and perception of treatment benefit. Neurology 85:498–504. https://doi.org/10.1212/wnl.0000000000001833
Alshekhlee A, Miles JD, Katirji B, Preston DC, Kaminski HJ (2009) Incidence and mortality rates of myasthenia gravis and myasthenic crisis in US hospitals. Neurology 72:1548–1554. https://doi.org/10.1212/WNL.0b013e3181a41211
Barth D, Nabavi Nouri M, Ng E, Nwe P, Bril V (2011) Comparison of IVIg and PLEX in patients with myasthenia gravis. Neurology 76:2017–2023. https://doi.org/10.1212/WNL.0b013e31821e5505
Bershad EM, Feen ES, Suarez JI (2008) Myasthenia gravis crisis. South Med J 101:63–69. https://doi.org/10.1097/SMJ.0b013e31815d4398
Bonnan M, Valentino R, Debeugny S, Merle H, Fergé JL, Mehdaoui H, Cabre P (2018) Short delay to initiate plasma exchange is the strongest predictor of outcome in severe attacks of NMO spectrum disorders. J Neurol Neurosurg Psychiatry 89:346–351. https://doi.org/10.1136/jnnp-2017-316286
Broadley J, Seneviratne U, Beech P, Buzzard K, Butzkueven H, O’Brien T, Monif M (2019) Prognosticating autoimmune encephalitis: A systematic review. J Autoimmun 96:24–34. https://doi.org/10.1016/j.jaut.2018.10.014
Brusaferri F, Candelise L (2000) Steroids for multiple sclerosis and optic neuritis: a meta-analysis of randomized controlled clinical trials. J Neurol 247:435–442. https://doi.org/10.1007/s004150070172
Cocito D et al (2010) A nationwide retrospective analysis on the effect of immune therapies in patients with chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol 17:289–294. https://doi.org/10.1111/j.1468-1331.2009.02802.x
Codron P, Cousin M, Subra JF, Pautot V, Letournel F, Verny C, Cassereau J (2017) Therapeutic plasma exchange in chronic dysimmune peripheral neuropathies: A 10-year retrospective study. J Clin Apher 32:413–422. https://doi.org/10.1002/jca.21530
Correia I et al (2018) Plasma exchange in severe acute relapses of multiple sclerosis - Results from a Portuguese cohort. Mult Scler Relat Disord 19:148–152. https://doi.org/10.1016/j.msard.2017.12.001
Cortese I, Chaudhry V, So YT, Cantor F, Cornblath DR, Rae-Grant A (2011) Evidence-based guideline update: plasmapheresis in neurologic disorders: report of the therapeutics and technology assessment subcommittee of the american academy of neurology. Neurol 76:294–300. https://doi.org/10.1212/WNL.0b013e318207b1f6
Dau PC (1995) Increased antibody production in peripheral blood mononuclear cells after plasma exchange therapy in multiple sclerosis. J Neuroimmunol 62:197–200. https://doi.org/10.1016/0165-5728(95)00121-4
Dau PC, Lindstrom JM, Cassel CK, Denys EH, Shev EE, Spitler LE (1977) Plasmapheresis and immunosuppressive drug therapy in myasthenia gravis. N Engl J Med 297:1134–1140. https://doi.org/10.1056/nejm197711242972102
DeSena AD et al (2015) Intravenous methylprednisolone versus therapeutic plasma exchange for treatment of anti-N-methyl-D-aspartate receptor antibody encephalitis: A retrospective review. J Clin Apher 30:212–216. https://doi.org/10.1002/jca.21363
Dispenzieri A (2005) POEMS syndrome. Hematol Am Soc Hematol Educ Program 2005:360–367. https://doi.org/10.1182/asheducation-2005.1.360
Dispenzieri A (2007) POEMS syndrome. Blood Rev 21:285–299. https://doi.org/10.1016/j.blre.2007.07.004
Dorst J, Ludolph AC, Senel M, Tumani H (2018) Short-term and long-term effects of immunoadsorption in refractory chronic inflammatory demyelinating polyneuropathy: a prospective study in 17 patients. J Neurol 265:2906–2915. https://doi.org/10.1007/s00415-018-9082-6
Dubey D et al (2018) Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol 83:166–177. https://doi.org/10.1002/ana.25131
Dyck PJB, Tracy JA (2018) History, diagnosis, and management of chronic inflammatory demyelinating polyradiculoneuropathy. Mayo Clin Proc 93:777–793. https://doi.org/10.1016/j.mayocp.2018.03.026
Elovaara I, Hietaharju A (2010) Can we face the challenge of expanding use of intravenous immunoglobulin in neurology? Acta Neurol Scand 122:309–315. https://doi.org/10.1111/j.1600-0404.2009.01317.x
Elovaara I, Lällä M, Spåre E, Lehtimäki T, Dastidar P (1998) Methylprednisolone reduces adhesion molecules in blood and cerebrospinal fluid in patients with MS. Neurology 51:1703–1708. https://doi.org/10.1212/wnl.51.6.1703
Etemadifar M, Nasr Z, Khalili B, Taherioun M, Vosoughi R (2015) Epidemiology of neuromyelitis optica in the world: a systematic review and meta-analysis. Mult Scler Int 2015:174720. https://doi.org/10.1155/2015/174720
Fassbender C, Klingel R, Köhler W (2017) Immunoadsorption for autoimmune encephalitis. Atheroscler Suppl 30:257–263. https://doi.org/10.1016/j.atherosclerosissup.2017.05.041
Fitzner D, Simons M (2010) Chronic progressive multiple sclerosis - pathogenesis of neurodegeneration and therapeutic strategies. Curr Neuropharmacol 8:305–315. https://doi.org/10.2174/157015910792246218
Gajdos P, Chevret S, Clair B, Tranchant C, Chastang C (1997) Clinical trial of plasma exchange and high-dose intravenous immunoglobulin in myasthenia gravis. Myasthenia Gravis Clinical Study Group. Ann Neurol 41:789–796. https://doi.org/10.1002/ana.410410615
Gajdos P, Chevret S, Toyka K (2002) Plasma exchange for myasthenia gravis. Cochrane Database Syst Rev: CD002275. https://doi.org/10.1002/14651858.cd002275
Galldiks N et al (2011) Immunoadsorption in patients with chronic inflammatory demyelinating polyradiculoneuropathy with unsatisfactory response to first-line treatment. Eur Neurol 66:183–189. https://doi.org/10.1159/000331011
Heine J, Ly LT, Lieker I, Slowinski T, Finke C, Pruss H, Harms L (2016) Immunoadsorption or plasma exchange in the treatment of autoimmune encephalitis: a pilot study. J Neurol 263:2395–2402. https://doi.org/10.1007/s00415-016-8277-y
Hoffmann S, Meisel A (2018) Escalation strategies in the treatment of refractory myasthenia gravis. Neurol Int Open 02:E56–E59. https://doi.org/10.1055/s-0043-125342
Iijima M et al (2008) Prevalence and incidence rates of chronic inflammatory demyelinating polyneuropathy in the Japanese population. J Neurol Neurosurg Psychiatry 79:1040–1043. https://doi.org/10.1136/jnnp.2007.128132
Keegan M et al (2005) Relation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet 366:579–582. https://doi.org/10.1016/s0140-6736(05)67102-4
Keegan M, Pineda AA, McClelland RL, Darby CH, Rodriguez M, Weinshenker BG (2002) Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology 58:143–146. https://doi.org/10.1212/WNL.58.1.143
Khatri BO, Kramer J, Dukic M, Palencia M, Verre W (2012) Maintenance plasma exchange therapy for steroid-refractory neuromyelitis optica. J Clin Apher 27:183–192. https://doi.org/10.1002/jca.21215
Kleiter I et al (2016) Neuromyelitis optica: evaluation of 871 attacks and 1,153 treatment courses. Ann Neurol 79:206–216. https://doi.org/10.1002/ana.24554
Kozanoglu I, Deniz Y, Buyukkurt NT, Yeral M, Bo a C, Ozdogu H (2015) A Retrospective study on patients with guillain-barré syndrome treated with therapeutic plasma exchange and other treatment options a centre s experience. Europ Neurol Rev 10:81
Kumar R, Birinder SP, Gupta S, Singh G, Kaur A (2015) Therapeutic plasma exchange in the treatment of myasthenia gravis. Indian J Crit Care Med 19:9–13. https://doi.org/10.4103/0972-5229.148631
Lehmann HC, Hartung HP (2011) Plasma exchange and intravenous immunoglobulins: mechanism of action in immune-mediated neuropathies. J Neuroimmunol 231:61–69. https://doi.org/10.1016/j.jneuroim.2010.09.015
Leray E, Moreau T, Fromont A, Edan G (2016) Epidemiology of multiple sclerosis. Rev Neurol 172:3–13. https://doi.org/10.1016/j.neurol.2015.10.006
Lieker I, Slowinski T, Harms L, Hahn K, Klehmet J (2017) A prospective study comparing tryptophan immunoadsorption with therapeutic plasma exchange for the treatment of chronic inflammatory demyelinating polyneuropathy. J Clin Apher 32:486–493. https://doi.org/10.1002/jca.21546
Linker RA, Gold R (2008) Use of intravenous immunoglobulin and plasma exchange in neurological disease. Curr Opin Neurol 21:358–365. https://doi.org/10.1097/WCO.0b013e3282ff5b8f
Lipphardt M et al (2019) Immunoadsorption or plasma exchange in steroid-refractory multiple sclerosis and neuromyelitis optica. J Clin Apher 34:381–391. https://doi.org/10.1002/jca.21686
Llufriu S et al (2009) Plasma exchange for acute attacks of CNS demyelination: Predictors of improvement at 6 months. Neurol 73:949–953. https://doi.org/10.1212/WNL.0b013e3181b879be
Lublin FD, Baier M, Cutter G (2003) Effect of relapses on development of residual deficit in multiple sclerosis. Neurol 61:1528–1532. https://doi.org/10.1212/01.wnl.0000096175.39831.21
Mackenzie IS, Morant SV, Bloomfield GA, MacDonald TM, O’Riordan J (2014) Incidence and prevalence of multiple sclerosis in the UK 1990–2010: a descriptive study in the General Practice Research Database. J Neurol Neurosurg Psychiatry 85:76–84. https://doi.org/10.1136/jnnp-2013-305450
Mahdi-Rogers M, Hughes RA (2014) Epidemiology of chronic inflammatory neuropathies in southeast England. Eur J Neurol 21:28–33. https://doi.org/10.1111/ene.12190
Makroo RN, Raina V, Kohli A, Suri V, Kumar P (2008) Effectiveness of Therapeutic Plasma Exchange in Myasthenia Gravis. Apollo Med 5:118-120. https://doi.org/10.1016/S0976-0016(11)60132-4
Makuch M et al (2018) N-methyl-D-aspartate receptor antibody production from germinal center reactions: Therapeutic implications. Ann Neurol 83:553–561. https://doi.org/10.1002/ana.25173
Mantegazza R, Antozzi C (2018) When myasthenia gravis is deemed refractory: clinical signposts and treatment strategies. Ther Adv Neurol Disord 11:1756285617749134. https://doi.org/10.1177/1756285617749134
McGrogan A, Sneddon S, de Vries CS (2010) The Incidence of Myasthenia Gravis: A Systematic Literature Review. Neuroepidemiol 34:171–183. https://doi.org/10.1159/000279334
McLeod JG, Pollard JD, Macaskill P, Mohamed A, Spring P, Khurana V (1999) Prevalence of chronic inflammatory demyelinating polyneuropathy in New South Wales, Australia. Ann Neurol 46:910–913. https://doi.org/10.1002/1531-8249(199912)46:6%3c910::AID-ANA14%3e3.0.CO;2-2
Mehndiratta MM, Hughes RA, Pritchard J (2015) Plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev:CD003906. https://doi.org/10.1002/14651858.CD003906.pub4
Miyamoto K, Kusunoki S (2009) Intermittent plasmapheresis prevents recurrence in neuromyelitis optica. Ther Apher Dial 13:505–508. https://doi.org/10.1111/j.1744-9987.2009.00780.x
Moser T et al (2019) Therapeutic Plasma Exchange in Multiple Sclerosis and Autoimmune Encephalitis: a Comparative Study of Indication. Efficacy and Safety Brain Sci. https://doi.org/10.3390/brainsci9100267
Mygland A, Monstad P (2001) Chronic polyneuropathies in Vest-Agder, Norway. Eur J Neurol 8:157–165. https://doi.org/10.1046/j.1468-1331.2001.00187.x
Narayanaswami P et al (2021) International Consensus Guidance for Management of Myasthenia Gravis: 2020 Update. Neurol 96:114–122. https://doi.org/10.1212/wnl.0000000000011124
Newsom-Davis J, Wilson SG, Vincent A, Ward CD (1979) Long-term effects of repeated plasma exchange in myasthenia gravis. Lancet 1:464–468. https://doi.org/10.1016/S0140-6736(79)90823-7
Oji S, Nomura K (2017) Immunoadsorption in neurological disorders. Transfus Apher Sci 56:671–676. https://doi.org/10.1016/j.transci.2017.08.013
Palace J, Leite MI, Jacob A (2012) A practical guide to the treatment of neuromyelitis optica. Prac Neurol 12:209–214. https://doi.org/10.1136/practneurol-2012-000237
Prytuła A et al (2015) Therapeutic plasma exchange in children with acute autoimmune central nervous system disorders. Int J Artif Organs 38:494–500. https://doi.org/10.5301/ijao.5000435
Ramanathan S, Al-Diwani A, Waters P, Irani SR (2019) The autoantibody-mediated encephalitides: from clinical observations to molecular pathogenesis. J Neurol. https://doi.org/10.1007/s00415-019-09590-9
Ramberger M et al (2020) Distinctive binding properties of human monoclonal LGI1 autoantibodies determine pathogenic mechanisms. Brain 143:1731–1745. https://doi.org/10.1093/brain/awaa104
Reeves HM, Winters JL (2014) The mechanisms of action of plasma exchange. Br J Haematol 164:342–351. https://doi.org/10.1111/bjh.12629
Rivner MH, Pasnoor M, Dimachkie MM, Barohn RJ, Mei L (2018) Muscle-Specific Tyrosine Kinase and Myasthenia Gravis Owing to Other Antibodies. Neurol Clin 36:293–310. https://doi.org/10.1016/j.ncl.2018.01.004
Rodriguez M, Karnes WE, Bartleson JD, Pineda AA (1993) Plasmapheresis in acute episodes of fulminant CNS inflammatory demyelination. Neurol 43:1100–1104. https://doi.org/10.1212/WNL.43.6.1100
Rubin DB, Batra A, Vaitkevicius H, Vodopivec I (2018) Autoimmune Neurologic Disorders. Am J Med 131:226–236. https://doi.org/10.1016/j.amjmed.2017.10.033
Ruprecht K, Klinker E, Dintelmann T, Rieckmann P, Gold R (2004) Plasma exchange for severe optic neuritis: treatment of 10 patients. Neurol 63:1081–1083. https://doi.org/10.1212/01.WNL.0000138437.99046.6B
Sanders DB et al (2016) International consensus guidance for management of myasthenia gravis: Executive summary. Neurol 87:419–425. https://doi.org/10.1212/wnl.0000000000002790
Agence nationale de sécurité du médicament et des produits de santé (2020) L’ANSM demande aux professionnels de santé de respecter la hiérarchisation des indications des immunoglobulines humaines normales (IgHN) - Point d'information. https://www.ansm.sante.fr/S-informer/Points-d-information-Points-d-information/L-ANSM-demande-aux-professionnels-de-sante-de-respecter-la-hierarchisation-des-indications-des-immunoglobulines-humaines-normales-IgHN-Point-d-information. Accessed 31 July 2020
Schilling S et al (2006) Plasma exchange therapy for steroid-unresponsive multiple sclerosis relapses: clinical experience with 16 patients. Nervenarzt 77:430–438. https://doi.org/10.1007/s00115-005-2019-1
Schroder A, Linker RA, Gold R (2009) Plasmapheresis for neurological disorders. Expert Rev Neurother 9:1331–1339. https://doi.org/10.1586/ern.09.81
Schwartz J et al (2016) Guidelines on the Use of Therapeutic Apheresis in Clinical Practice-Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Seventh Special Issue. J Clin Apher 31:149-162. https://doi.org/10.1002/jca.21470
Sellebjerg F, Christiansen M, Jensen J, Frederiksen JL (2000) Immunological effects of oral high-dose methylprednisolone in acute optic neuritis and multiple sclerosis. Eur J Neurol 7:281–289. https://doi.org/10.1046/j.1468-1331.2000.00074.x
Shin YW, Lee ST, Park KI, Jung KH, Jung KY, Lee SK, Chu K (2018) Treatment strategies for autoimmune encephalitis. Ther Adv Neurol Disord 11:1756285617722347. https://doi.org/10.1177/1756285617722347
Silvestri NJ, Wolfe GI (2014) Treatment-refractory myasthenia gravis. J Clin Neuromuscul Dis 15:167–178. https://doi.org/10.1097/cnd.0000000000000034
Stork L, Ellenberger D, Beissbarth T, Friede T, Lucchinetti CF, Bruck W, Metz I (2018) Differences in the reponses to apheresis therapy of patients with 3 histopathologically classified immunopathological patterns of multiple sclerosis. JAMA Neurol 75:428–435. https://doi.org/10.1001/jamaneurol.2017.4842
Suppiej A et al (2016) Plasma exchange in pediatric anti-NMDAR encephalitis: A systematic review. Brain Dev 38:613–622. https://doi.org/10.1016/j.braindev.2016.01.009
Thompson J et al (2018) The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain 141:348–356. https://doi.org/10.1093/brain/awx323
Titulaer MJ et al (2013) Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 12:157–165. https://doi.org/10.1016/s1474-4422(12)70310-1
Trebst C et al (2014) Update on the diagnosis and treatment of neuromyelitis optica: recommendations of the Neuromyelitis Optica Study Group (NEMOS). J Neurol 261:1–16. https://doi.org/10.1007/s00415-013-7169-7
Watanabe S, Nakashima I, Misu T, Miyazawa I, Shiga Y, Fujihara K, Itoyama Y (2007) Therapeutic efficacy of plasma exchange in NMO-IgG-positive patients with neuromyelitis optica. Mult Scler 13:128–132. https://doi.org/10.1177/1352458506071174
Weinshenker BG et al (1999) A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol 46:878–886. https://doi.org/10.1002/(SICI)1098-1101(1999)14:3%3c144::AID-JCA7%3e3.0.CO;2-R
Wilson R et al (2018) Condition-dependent generation of aquaporin-4 antibodies from circulating B cells in neuromyelitis optica. Brain 141:1063–1074. https://doi.org/10.1093/brain/awy010
Winklmeier S et al (2019) Identification of circulating MOG-specific B cells in patients with MOG antibodies. Neurol Neuroimmunol Neuroinflammation 6:625. https://doi.org/10.1212/NXI.0000000000000625
Wright S et al (2015) N-methyl-D-aspartate receptor antibody-mediated neurological disease: results of a UK-based surveillance study in children. Arch Dis Child 100:521–526. https://doi.org/10.1136/archdischild-2014-306795
Zhang Y, Huang HJ, Chen WB, Liu G, Liu F, Su YY (2021) Clinical efficacy of plasma exchange in patients with autoimmune encephalitis. Ann Clin Transl Neurol 8:763–773. https://doi.org/10.1002/acn3.51313
Acknowledgements
The authors would like to acknowledge Joke Vandewalle (Modis c/o Terumo BCT) for writing support, and Sophie Timmery (Modis c/o Terumo BCT) for editing and coordination support. The authors would like to acknowledge Prof. Mike Lunn for fruitful discussions on the topic and for his presentation at the 2018 Midlands Neurological Society meeting on PLEX in refractory neurology.
Funding
The development (writing, editing and coordination) of this manuscript was funded by Terumo BCT.
Author information
Authors and Affiliations
Contributions
All authors contributed to the development of this manuscript, including the literature search. They all approved the final content and submission to the journal.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest/Competing Interests
Anna Yudina is an employee of Terumo BCT (Belgium) and holds shares in Terumo BCT. Saiju Jacob reports advisory board remunerations from Alexion Pharmaceuticals, Alnylam Pharmaceuticals and ArgenX Ltds, and speaker/chairman fees from Eisai Ltd, UCB Pharma and Terumo BCT. This includes speaker fees received for the 2018 Midlands Neurological Society meeting on PLEX in refractory neurology, which is the basis of this article. Gordon Mazibrada reports advisory board consultancy honoraria, international meeting sponsorship and MS research and service development donations from Biogen, Genzyme, Novartis, Merck-Serono, Roche and Teva. This includes speaker fees received for the 2018 Midlands Neurological Society meeting on PLEX in refractory neurology, which is the basis of this article. Sarosh Irani reports grants, personal fees and non-financial support from UCB, personal fees from Roivant, personal fees and non-financial support from MedImmun, during the conduct of the study; personal fees from ADCT, grants from ONO, grants from CSL Behring, outside the submitted work; In addition, Sarosh Irani has a patent VGKC / LGI1/ CASPR2 antibodies with royalties. Anu Jacob reports honoraria and speaker fees from Terumo BCT for talks related to plasma exchange. Anu Jacob is lead for the UK NHS NMO service (2010-2020).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jacob, S., Mazibrada, G., Irani, S.R. et al. The Role of Plasma Exchange in the Treatment of Refractory Autoimmune Neurological Diseases: a Narrative Review. J Neuroimmune Pharmacol 16, 806–817 (2021). https://doi.org/10.1007/s11481-021-10004-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-021-10004-9