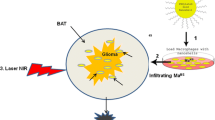
Abstract
Gold based nanoparticles with strong near infra-red (NIR) absorption are ideally suited for photothermal therapy (PTT) of brain tumors. The goal of PTT is to induce rapid heating in tumor tissues while minimizing thermal diffusion to normal brain. PTT efficacy is sensitively dependent on both nanoparticle concentration and distribution in tumor tissues. Nanoparticle delivery via passive approaches such as the enhanced permeability and retention (EPR) effect is unlikely to achieve sufficient nanoparticle concentrations throughout tumor volumes required for effective PTT. A simple approach for improving tumor biodsitribution of nanoparticles is the use of cellular delivery vehicles. Specifically, this review focuses on the use of monocytes/macrophages (Mo/Ma) as gold nanoparticle delivery vectors for PTT of brain tumors. Although the efficacy of this delivery approach has been demonstrated in both in vitro and animal PTT studies, its clinical potential for the treatment of brain tumors remains uncertain.



Similar content being viewed by others
References
Akhavan O, Ghaderi E (2013) Graphene nanomesh promises extremely efficient in vivo photothermal therapy. Small 9(21):3593–3601
Akhavan O, Ghaderi E, Akhavan A (2012) Size-dependent genotoxicity of graphene nanoplatelets in human stem cells. Biomaterials 33(32):8017–8025
Aslan K, Lacowicz J, Geddes CR (2005) Plasmon light scattering in biology and medicine: new sensing approaches, visions and perspectives. Curr Opin Chem Biol 9(5):1367–5931
Badie B, Schartner JM (2000) Flow cytometric characterization of tumor associated macrophages in experimental gliomas. Neurosurgery 46:957–961
Baek SK, Makkouk AR, Krasieva T, Sun CH, Madsen SJ, Hirschberg H (2011) Photothermal treatment of glioma; an in vitro study of macrophage-mediated delivery of gold nanoshells. J Neurooncol 104(2):439–448
Barua S, Mitragotri S (2014) Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today 9:223–243
Basel MT, Shrestha TB, Bossmann SH, Troyer DL (2014) Cells as delivery vehicles for cancer therapeutics. Ther Deliv 5(5):555–567
Cai W, Gao T, Hong H, Sun J (2008) Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol Sci Appl 1:17–32
Carolina A, da Fonseca C, Badie B (2013) Microglia and macrophages in malignant gliomas: recent discoveries and implications for promising therapies. Clin Dev Immunol 2013:265608
Chen J, Glaus C, Laforest R, Zhang Q, Yang M, Gidding M, Welch MJ, Xia Y (2010) Gold nanocages as photothermal transducers for cancer treatment. Small 6(7):811–817
Chhetri S, Hirschberg H, Madsen SJ (2014) Photothermal therapy of human glioma spheroids with gold-silica nanoshells and gold nanorods: a comparative study. Proceedings SPIE 8928:U1–U8
Choi MR, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JP, Bashir R, Halas NJ, Clare SE (2007) A cellular Trojan horse for delivery of therapeutic nanoparticles into tumors. Nano Lett 7(12):3759–3765
Christie C, Madsen SJ, Peng Q, Hirschberg H (2015) Macrophages as nanoparticle delivery vectors for photothermal therapy of brain tumors. Ther Deliv 6(3):371–384
Cole JR, Mirin NA, Knight MW, Goodrich GP, Halas NJ (2009) Photothermal efficiencies of nanoshells and nanorods for clinical therapeutic applications. J Phys Chem C 113(28):12090–12094
Cuenca AG, Jiang H, Hochwald SN, Delano M, Cance WG, Grobmyer SR (2006) Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer 107(3):459–466
Day ES, Thompson PA, Zhang L, Lewinski NA, Ahmed N, Drezek RA, Blaney SM, West JL (2011) Nanoshell-mediated photothermal therapy improves survival in a murine glioma model. J Neurooncol 104:55–63
Day ES, Zhang L, Thompson PA, Zawaski JA, Kaffes CC, Gaber MW, Blaney SM, West JL (2012) Vascular-targeted photothermal therapy of an orthotopic murine glioma model. Nanomedicine (London) 7(8):1133–1148
Fischbach MA, Bluestone JA, Lim WA (2013) Sci tCell-based therapeutics: the next pillar of medicine. Sci Transl Med 5(179):1–6
Hirsch LR, Gobin AM, Lowery AR, Tam F, Halas NJ (2006) Metal nanoshells. Ann Biomed Eng 334(1):15–22
Hirschberg H, Uzal FA, Chighvinadze D, Zhang MJ, Peng Q, Madsen SJ (2008) Disruption of the blood-brain barrier following ALA-mediated photodynamic therapy. Lasers Surg Med 40:535–542
Hirschberg H, Baek SK, Kwon YJ, Sun CH, Madsen SJ (2010) Bypassing the blood brain barrier: delivery of therapeutic agents by macrophages. Proceedings SPIE 7548:3Z1–3Z5
Huang X, El-Sayed IH, Qian W, El-Sayed MA (2006) Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc 128(6):2115–2120
Huang X, Jain PK, El-Sayed IH, El-Sayed MA (2008) Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci 23(3):217–228
Huynh E, Zheng G (2015) Cancer nanomedicine: addressing the dark side of the enhanced permeability and retention effect. Nanomed (Lond) 10(13):1993–1995
Jain PK, El Sayed MA (2007) Universal scaling of plasmon coupling in metal nanostructures: extension from particle pairs to nanoshells. Nano Lett 7:2854–2858
Kah JC, Wong KY, Neoh KG, Song JH, Fu JW, Mhaisalkar S, Olivo M, Sheppard CJ (2009) Critical parameters in the pegylation of gold nanoshells for biomedical applications: an in vitro macrophage study. J Drug Target 17:181–193
Kennedy LC, Bickford LR, Lewinski NA, Coughlin AJ, Hu Y, Day ES, West JL, Drezek RA (2011) A new era for cancer treatment: gold-nanoparticle-mediated thermal therapies. Small 7(2):169–183
Lin AW, Lewinski NA, West JL, Halas NJ, Drezek RA (2005) Optically tunable nanonoparticle contrast agents for early cancer deterction: Midel-based analysis of gold nanoshells. J Biomed Opt 10(6):064035
Link S, El-Sayed MA (1999) Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J Phys Chem B 103:4212–4217
Link S, El-Sayed MA (2003) Optical properties and ultrafast dynamics of metallic nanocrystals. Annu Rev Phys Chem 54:331–366
Liu C, Mi CC, Li BQ (2008) Energy absorption of gold nanoshells in hyperthermia therapy. IEEE Trans Nanobioscience 7(3):206–214
Loo C, Lin A, Hirsch L, Lee MH, Halas N, West J, Drezek R (2004) Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol Cancer Res Treat 3(1):33–44
Madsen SJ, Sun CH, Tromberg BJ, Cristine V, DeMagalhaes N, Hirschberg H (2006) Multicell tumor spheroids in photodynamic therapy. Lasers Surg Med 38:555–564
Madsen SJ, Gach HM, Hong SJ, Uzal FA, Peng Q, Hirschberg H (2013) Increased nanoparticle-loaded exogenous macrophage migration into the brain following PDT-induced blood-brain barrier disruption. Lasers Surg Med 45(8):524–532
Madsen SJ, Christie C, Hong SJ, Trinidad A, Chhetri S, Peng Q, Uzal FA, Hirschberg H (2015) Nanoparticle-loaded macrophage-mediated photothermal therapy: potential for glioma treatment. Lasers Med Sci 30(4):1357–1365
Madsen SJ, Shih EC, Peng Q, Christie C, Krasieva T, Hirschberg H (2016) Photothermal enhancement of chemotherapy mediated by gold-silica nanoshell-loaded macrophages: in vitro squamous cell carcinoma study. J Biomed Opt 21(1):018004
Maeda H (2001) The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul 41(1):189–207
Maeda H (2015) Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev 91:1–152
Mathews MS, Chighvinadze D, Gach HM, Uzal FA, Madsen SJ, Hirschberg H (2011) Cerebral edema following photodynamic therapy using endogenous and exogenous photosensitizers in normal brain. Lasers Surg Med 43:892–900
Oldenburg SJ, Averitt RD, Westcott SL, Halas NJ (1998) Nanoengineering of optical resonances. Chem Phys Lett 288:243–247
Petrecca K, Guiot MC, Panet-Raymond V (2013) Failure pattern following complete resection plus radiotherapy and temozolomide is at the resection margin in patients with Glioblastoma. J Neurooncol 111:19–23
Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC (2013) Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res 73(8):2412–2417
Prodan E, Radioff C, Halas NJ, Nordlander PA (2003) A hybridization model for the plasmon response of complex nanostructures. Science 301:419–422
Robinson JT, Tabakman SM, Liang Y, Wang H, Casalongue HS, Vinh D, Dai H (2011) Ultrasmall reduced grapheme oxide with high near-infrared absorbance for photothermal therapy. J Am Chem Soc 133(17):6825–6831
Roggendorf W, Strupp S, Paulus W (1996) Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathol 92:288–293
Schwartz JA, Shetty AM, Price RE, Stafford RJ, Wang JC, Uthamanthil RK, Pham K, McNichols RJ, Coleman CL, Payne JD (2009) Feasibility study of particle-assisted laser ablation of brain tumors in orthotopic canine model. Cancer Res 69(4):1659–1667
Terentyuk GS, Maslyakova GN, Suleymanova LV, Khlebtsov NG, Khlebtsov BN, Akchurin GG (2009) Laser-induced tissue hyperthermia mediated by gold nanoparticles: toward cancer phototherapy. J Biomed Opt 14(2):021016
Trédan O, Galmarini CM, Patel K, Tannock IF (2007) Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 99(19):1441–1454
Trinidad A, Hong SJ, Peng Q, Madsen SJ, Hirschberg H (2014) Combined concurrent photodynamic and gold nanoshell loaded macrophage-mediated photothermal therapies: an in vitro study on squamous cell head and neck carcinoma. Lasers Surg Med 46(4):310–318
Valable S, Barbier EL, Bernaudin M, Roussel S, Segebarth C, Petit E, Remy C (2008) In vivo MRI tracking of exogenous monocytes/macrophages targeting brain tumors in a rat model of glioma. Neuroimage 40(2):973–983
Wang Y, Wang K, Zhao J, Liu X, Bu J, Yan X, Huang R (2013) Multifunctional mesoporous silica-coated grapheme nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. J Am Chem Soc 135:4799–4804
Wu J, Yang S, Luo H, Zeng L, Ye L, Lu Y (2006) Quantitative evaluation of monocyte transmigration into the brain following chemical opening of the blood-brain barrier in mice. Brain Res 1098:79–85
Yang TD, Choi W, Yoon TH, Lee KJ, Lee JS, Jang HJ, Lee MG, Yim HS, Choi KM, Kim B, Lee JJ, Kim H, Lee DY, Jung KY, Baek SK (2015) In vivo photothermal treatment by the peritumoral injection of macrophages loaded with gold nanoshells. Biomed Opt Express 7(1):185–193
Yi L, Xiao H, Xu M, Ye X, Hu J, Li F, Li M, Luo C, Yu S, Bian X, Feng H (2011) Glioma-initiating cells: a predominant role in microglia/macrophages tropism to glioma. J Neuroim 232(1–2):75–82
Acknowledgments
The authors are grateful for support from the Norwegian Radium Hospital Research Foundation. Portions of this work were made possible through access to the LAMMP Program NIBIB P41EB015890 at UCI. Steen Madsen was supported, in part, by the Tony and Renee Marlon Charitable Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hirschberg, H., Madsen, S.J. Cell Mediated Photothermal Therapy of Brain Tumors. J Neuroimmune Pharmacol 12, 99–106 (2017). https://doi.org/10.1007/s11481-016-9690-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-016-9690-9