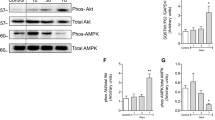
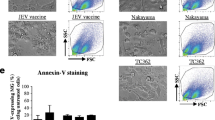
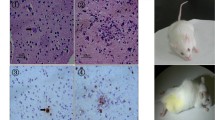
Abstract
Inflammation in the central nervous system (CNS) in Japanese encephalitis (JE) is shown to be the result of microglial activation that leads to the release of various proinflammatory mediators. Peripheral macrophages have been reported to infiltrate into the CNS in JE, though their contribution to the inflammatory process is yet to be elucidated. In this study, using an in vitro macrophage model, we have shown that upon JE virus infection, these cells secrete various soluble factors which may significantly add to the existing inflammatory milieu and lead to apoptotic or necrotic death of neurons. However, it is difficult to quantify the extent of involvement of either the microglia or infiltrating macrophages in the inflammatory processes.








Similar content being viewed by others
References
Aktas O, Ullrich O, Infante-Duarte C, Nitsch R, Zipp F (2007) Neuronal damage in brain inflammation. Arch Neurol 64:185–189
Aleyas AG, George JA, Han YW, Rahman MM, Kim SJ, Han SB, Kim BS, Kim K, Eo SK (2009) Functional modulation of dendritic cells and macrophages by Japanese encephalitis virus through MyD88 adaptor molecule-dependent and -independent pathways. J Immunol 183:2462–2474
Appaiahgari MB, Vrati S (2007) DNAzyme-mediated inhibition of Japanese encephalitis virus replication in mouse brain. Mol Ther 15:1593–1599
Appaiahgari MB, Saini M, Rauthan M, Jyoti VS (2006) Immunization with recombinant adenovirus synthesizing the secretory form of Japanese encephalitis virus envelope protein protects adenovirus-exposed mice against lethal encephalitis. Microbes Infect 8:92–104
Araki E, Forster C, Dubinsky JM, Ross ME, Iadecola C (2001) Cyclooxygenase-2 inhibitor ns-398 protects neuronal cultures from lipopolysaccharide-induced neurotoxicity. Stroke 32:2370–2375
Basini G, Grasselli F, Bianco F, Tirelli M, Tamanini C (2004) Effect of reduced oxygen tension on reactive oxygen species production and activity of antioxidant enzymes in swine granulosa cells. Biofactors 20:61–69
Bergamini S, Rota C, Canali R, Staffieri M, Daneri F, Bini A, Giovannini F, Tomasi A, Iannone A (2001) N-acetylcysteine inhibits in vivo nitric oxide production by inducible nitric oxide synthase. Nitric Oxide 5:349–360
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC (2006) CNS immune privilege: hiding in plain sight. Immunol Rev 213:48–65
Chan ED, Winston BW, Uh S-T, Wynes MW, Rose DM, Riches DWH (1999) Evaluation of the role of mitogen-activated protein kinases in the expression of inducible nitric oxide synthase by IFN-γ and TNF-α in mouse macrophages. J Immunol 162:415–422
Chen CJ, Chen JH, Chen SY, Liao SL, Raung SL (2004) Upregulation of RANTES gene expression in neuroglia by Japanese encephalitis virus infection. J Virol 78:12107–12119
Chen CJ, Ou YC, Lin SY, Raung SL, Liao SL, Lai CY, Chen SY, Chen JH (2010) Glial activation involvement in neuronal death by Japanese encephalitis virus infection. J Gen Virol 91:1028–1037
Crofford LJ (1997) COX-1 and COX-2 tissue expression: implications and predictions. J Rheumatol Suppl 49:15–19
Diamond MS (2003) Evasion of innate and adaptive immunity by flaviviruses. Immunol Cell Biol 81:196–206
Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE (1998) Cyclooxygenase in biology and disease. FASEB J 12:1063–1073
Dutta K, Ghosh D, Basu A (2009) Curcumin protects neuronal cells from Japanese encephalitis virus-mediated cell death and also inhibits infective viral particle formation by dysregulation of ubiquitin–proteasome system. J Neuroimmune Pharmacol 4:328–337
Dutta K, Rangarajan PN, Vrati S, Basu A (2010a) Japanese encephalitis: pathogenesis, prophylactics and therapeutics. Curr Sci 98:22–30
Dutta K, Mishra MK, Nazmi A, Kumawat KL, Basu A (2010b) Minocycline differentially modulates macrophage mediated peripheral immune response following Japanese encephalitis virus infection. Immunobiology 215:884–893
Dutta K, Ghosh D, Nazmi A, Kumawat KL, Basu A (2010c) A common carcinogen benzo[a]pyrene causes neuronal death in mouse via microglial activation. PLoS ONE 5:e9984
Dutta K, Kumawat KL, Nazmi A, Mishra MK, Basu A (2010d) Minocycline differentially modulates viral infection and persistence in an experimental model of Japanese encephalitis. J Neuroimmune Pharmacol 5:553–565
Flavin MP, Coughlin K, Ho LT (1997) Soluble macrophage factors trigger apoptosis in cultured hippocampal neurons. Neuroscience 80:437–448
Ghoshal A, Das S, Ghosh S, Mishra MK, Sharma V, Koli P, Sen E, Basu A (2007) Proinflammatory mediators released by activated microglia induces neuronal death in Japanese encephalitis. Glia 55:483–496
Hui KP, Lee SM, Cheung CY, Ng IH, Poon LL, Guan Y, Ip NY, Lau AS, Peiris JS (2009) Induction of proinflammatory cytokines in primary human macrophages by influenza A virus (H5N1) is selectively regulated by IFN regulatory factor 3 and p38 MAPK. J Immunol 182:1088–1098
Kimura T, Iwase M, Kondo G, Watanabe H, Ohashi M, Ito D, Nagumo M (2003) Suppressive effect of selective cyclooxygenase-2 inhibitor on cytokine release in human neutrophils. Int Immunopharmacol 3:1519–1528
Kobayashi Z, Tsuchiya K, Yokota O, Haga C, Arai T, Akiyama H, Kotera M, Mizusawa H (2009) Japanese encephalitis—serial CT findings and neuropathology in an autopsy case. Clin Neuropathol 28:422–429
Lappas M, Permezel M, Rice GE (2003) N-acetyl-cysteine inhibits phospholipid metabolism, proinflammatory cytokine release, protease activity, and nuclear factor-kappaB deoxyribonucleic acid-binding activity in human fetal membranes in vitro. J Clin Endocrinol Metab 88:1723–1729
Lin YL, Huang YL, Ma SH, Yeh CT, Chiou SY, Chen LK, Liao CL (1997) Inhibition of Japanese encephalitis virus infection by nitric oxide: antiviral effect of nitric oxide on RNA virus replication. J Virol 71:5227–5235
Lindenbach BD, Rice CM (2003) Molecular biology of flaviviruses. Adv Virus Res 59:23–61
Mathur A, Bharadwaj M, Kulshreshtha R, Rawat S, Jain A, Chaturvedi UC (1988) Immunopathological study of spleen during Japanese encephalitis virus infection in mice. Br J Exp Pathol 69:423–432
Mathur A, Khanna N, Chaturvedi UC (1992) Breakdown of blood–brain barrier by virus-induced cytokine during Japanese encephalitis virus infection. Int J Exp Pathol 73:603–611
Matsuda K, Nakamura S, Matsushita T (2006) Celecoxib inhibits nitric oxide production in chondrocytes of ligament-damaged osteoarthritic rat joints. Rheumatol Int 26:991–995
Miller DW (1999) Immunobiology of the blood–brain barrier. J Neurovirol 5:570–578
Mishra MK, Basu A (2008) Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J Neurochem 105:1582–1595
Mishra MK, Ghosh D, Duseja R, Basu A (2009a) Antioxidant potential of minocycline in Japanese encephalitis virus infection in murine neuroblastoma cells: correlation with membrane fluidity and cell death. Neurochem Int 54:464–470
Mishra MK, Dutta K, Saheb SK, Basu A (2009b) Understanding the molecular mechanism of blood–brain barrier damage in an experimental model of Japanese encephalitis: correlation with minocycline administration as a therapeutic agent. Neurochem Int 55:717–723
Morimoto RI, Santoro MG (1998) Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol 16:833–838
Nagaoka U, Kim K, Jana NR, Doi H, Maruyama M, Mitsui K, Oyama F, Nukina N (2004) Increased expression of p62 in expanded polyglutamine-expressing cells and its association with polyglutamine inclusions. J Neurochem 91:57–68
Nunoshiba T, deRojas-Walker T, Wishnok JS, Tannenbaum SR, Demple B (1993) Activation by nitric oxide of an oxidative-stress response that defends Escherichia coli against activated macrophages. Proc Natl Acad Sci USA 90:9993–9997
Pulliam L, Herndier BG, Tang NM, McGrath MS (1991) Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alterations in cultured human brains. J Clin Invest 87:503–512
Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW (1999) Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res Brain Res Rev 30:77–105
Rose JW, Hill KE, Wada Y, Kurtz CI, Tsunoda I, Fujinami RS, Cross AH (1998) Nitric oxide synthase inhibitor, aminoguanidine, reduces inflammation and demyelination produced by Theiler’s virus infection. J Neuroimmunol 81:82–89
Strauss KI (2008) Antiinflammatory and neuroprotective actions of COX2 inhibitors in the injured brain. Brain Behav Immun 22:285–298
Swarup V, Ghosh J, Mishra MK, Basu A (2008) Novel strategy for treatment of Japanese encephalitis using arctigenin, a plant lignan. J Antimicrob Chemother 61:679–688
Tak PP, Firestein GS (2001) NF-kappaB: a key role in inflammatory diseases. J Clin Invest 107:7–11
Trifilieff A, Fujitani Y, Mentz F, Dugas B, Fuentes M, Bertrand C (2000) Inducible nitric oxide synthase inhibitors suppress airway inflammation in mice through down-regulation of chemokine expression. J Immunol 165:1526–1533
Wang S, Zhu H, Chen C (2000) Reactive oxygen species contribute to the induction of superoxide dismutase during heat shock in cultured rat neonatal cardiomyocytes. Chin Med J (Engl) 113:606–609
Yang KD, Yeh WT, Chen RF, Chuon HL, Tsai HP, Yao CW, Shaio MF (2004) A model to study neurotropism and persistency of Japanese encephalitis virus infection in human neuroblastoma cells and leukocytes. J Gen Virol 85:635–642
Yasui K (2002) Neuropathogenesis of Japanese encephalitis virus. J Neurovirol 8(Suppl 2):112–114
Zhang RL, Chopp M, Zaloga C, Zhang ZG, Jiang N, Gautam SC, Tang WX, Tsang W, Anderson DC, Manning AM (1995) The temporal profiles of ICAM-1 protein and mRNA expression after transient MCA occlusion in the rat. Brain Res 682:182–188
Acknowledgements
The authors would like to thank Vinod Babu for his help and Manish Kumar Dogra and Kanhaiya Lal Kumawat for their technical assistance.
Financial disclosure
This work is funded by a grant from the Council of Scientific and Industrial Research (CSIR), Govt. of India [27(1638)/10] to A.B. A.N. is a recipient of the Junior Research Fellowship from the Council of Scientific and Industrial Research, Government of India; K.D. is a recipient of a Research Associateship in Biotechnology and Life Sciences from the Department of Biotechnology, Government of India; S.D. is a recipient of a Senior Research Fellowship from University Grants Commission, Government of India.
Conflict of interest
The authors declare that there are no conflicts of interests with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Internalization and persistence of JEV within macrophages. Immunocytochemical staining of mock-infected and JEV-infected primary macrophages with CD11b and JEV antigen showed that the virus was present within the macrophages even after 48 h post infection. Magnification ×40; scale bar corresponds to 50 μm (GIF 132 kb)
Fig. S2
Plaque assay performed with conditioned media obtained from JEV-infected macrophages showed no visible cytopathic effect on a monolayer of Vero cells at various dilutions. Following incubation with the conditioned media, Vero cell monolayers were stained with crystal violet solution. However, no plaque formation could be visualized (GIF 8 kb)
Fig. S3
After 12 h of infection with JEV (J), the release of IL-12p70, MCP-1, TNF-α, IL-6 and IFN-γ (a–e) were significantly increased from macrophages. Application of NFκB and p38MAPK inhibitors MG-132 and SKF-86002 onto JEV-infected macrophages (J+MG and J+S, respectively) resulted in marked reduction in their levels (*p < 0.01 for J compared to mock-infected; # p < 0.01 for J+MG and J+S compared to J only) (GIF 39 kb)
Fig. S4
Immunoblot showing Bcl-2 and Bax levels in N2a that were treated with 12-h post-incubation conditioned media from mock-infected (M), JEV-infected (J), JEV-infected and MG-132 treated (J+MG) and JEV-infected and SKF-86002-treated (J+S) macrophages (a). Bcl-2 levels were significantly increased and Bax levels were decreased, following the addition of media from J+MG and J+S macrophages, in comparison to those treated with conditioned media from macrophages that were infected with JEV only (*p < 0.01 for J compared to mock-infected; # p < 0.01 for J+MG and J+S compared to J only) (GIF 35 kb)
Rights and permissions
About this article
Cite this article
Nazmi, A., Dutta, K., Das, S. et al. Japanese Encephalitis Virus-Infected Macrophages Induce Neuronal Death. J Neuroimmune Pharmacol 6, 420–433 (2011). https://doi.org/10.1007/s11481-011-9271-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-011-9271-x