Abstract
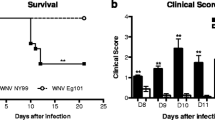
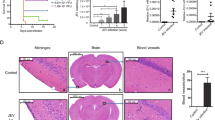
Japanese encephalitis (JE) is caused by a neurotropic flavivirus that causes CNS damage that leads to death in acute cases or permanent neuropsychiatric sequel in survivors. The course of infection of this virus is not well defined though it is clear that it evades the host's innate immune response in the periphery. The current study was designed to investigate the time-dependent changes in the spleen and lymph node, apart from the CNS that are infected by the Japanese encephalitis virus (JEV). Our previous studies have led to the identification of minocycline, a semi-synthetic antibiotic, as a protective drug in JE. In this study we have also investigated the role of minocycline on the peripheral organs that are infected by JEV. Levels of IL-12 and MCP-1 in the organs were estimated by cytometric bead array, and immunohistochemical studies were performed on cryosections of tissue to detect CD3- or CD11b-positive cells as well as JEV antigen. We found that the levels of T cell-activating cytokine IL-12 and MCP-1 levels were significantly elevated in JEV-infected tissue samples in a time-dependent manner. Corresponding to this increase was the increase in the number of CD3- and CD11b-positive cells in the tissues of infected animals. Minocycline treatment abrogated these changes. Minocycline treatment also resulted in the gradual decrease in the number of CD11b (but not CD3) positive cells in the lymph node and spleen, even though the virus persisted in these organs. We also observed structural changes in the spleen following minocycline treatment.







Similar content being viewed by others
References
Aleyas AG, George JA, Han YW, Rahman MM, Kim SJ, Han SB, Kim BS, Kim K, Eo SK (2009) Functional modulation of dendritic cells and macrophages by Japanese encephalitis virus through MyD88 adaptor molecule-dependent and -independent pathways. J Immunol 183:2462–2474
Boonpucknavig S, Bhamarapravati N, Nimmannitya S, Phalavadhtana A, Siripont J (1976) Immunofluorescent staining of the surfaces of lymphocytes in suspension from patients with dengue hemorrhagic fever. Am J Pathol 85:37–48
Chen LK, Liao CL, Lin CG, Lai SC, Liu CI, Ma SH, Huang YY, Lin YL (1996) Persistence of Japanese encephalitis virus is associated with abnormal expression of the nonstructural protein NS1 in host cells. Virology 217:220–229
Diamond MS (2003) Evasion of innate and adaptive immunity by flaviviruses. Immunol Cell Biol 81:196–206
Dutta K, Mishra MK, Nazmi A, Kumawat KL, Basu A (2010) Minocycline differentially modulates macrophage mediated peripheral immune response following Japanese encephalitis virus infection. Immunobiology (in press)
Hall WC, Crowell TP, Watts DM, Barros VL, Kruger H, Pinheiro F, Peters CJ (1991) Demonstration of yellow fever and dengue antigens in formalin-fixed paraffin-embedded human liver by immunohistochemical analysis. Am J Trop Med Hyg 45:408–417
Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML (1997) IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol 159:28–35
King AD, Nisalak A, Kalayanrooj S, Myint KS, Pattanapanyasat K, Nimmannitya S, Innis BL (1999) B cells are the principal circulating mononuclear cells infected by dengue virus. Southeast Asian J Trop Med Public Health 30:718–728
Kloppenburg M, Brinkman BM, de Rooij-Dijk HH, Miltenburg AM, Daha MR, Breedveld FC, Dijkmans BA, Verweij C (1996) The tetracycline derivative minocycline differentially affects cytokine production by monocytes and T lymphocytes. Antimicrob Agents Chemother 40:934–940
Kyle JL, Beatty PR, Harris E (2007) Dengue virus infects macrophages and dendritic cells in a mouse model of infection. J Infect Dis 195:1808–1817
Mathur A, Arora KL, Chaturvedi UC (1982) Transplacental Japanese encephalitis virus (JEV) infection in mice during consecutive pregnancies. J Gen Virol 59:213–217
Mathur A, Arora KL, Rawat S, Chaturvedi UC (1986) Persistence, latency and reactivation of Japanese encephalitis virus infection in mice. J Gen Virol 67:381–385
Mi W, Belyavskyi M, Johnson RR, Sieve AN, Storts R, Meagher MW, Welsh CJ (2004) Alterations in chemokine expression following Theiler's virus infection and restraint stress. J Neuroimmunol 151:103–115
Miagostovich MP, Ramos RG, Nicol AF, Nogueira RM, Cuzzi-Maya T, Oliveira AV, Marchevsky RS, Mesquita RP, Schatzmayr HG (1997) Retrospective study on dengue fatal cases. Clin Neuropathol 16:204–208
Mishra MK, Basu A (2008) Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J Neurochem 105:1582–1595
Mishra MK, Dutta K, Saheb SK, Basu A (2009a) Understanding the molecular mechanism of blood-brain barrier damage in an experimental model of Japanese encephalitis: correlation with minocycline administration as a therapeutic agent. Neurochem Int 55:717–723
Mishra MK, Ghosh D, Duseja R, Basu A (2009b) Antioxidant potential of minocycline in Japanese encephalitis virus infection in murine neuroblastoma cells: correlation with membrane fluidity and cell death. Neurochem Int 54:464–470
Omata N, Yasutomi M, Yamada A, Iwasaki H, Mayumi M, Ohshima Y (2002) Monocyte chemoattractant protein-1 selectively inhibits the acquisition of CD40 ligand-dependent IL-12-producing capacity of monocyte-derived dendritic cells and modulates Th1 immune response. J Immunol 169:4861–4866
Randolph GJ, Angeli V, Swartz MA (2005) Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol 5:617–628
Roy EJ, Gawlick U, Orr BA, Rund LA, Webb AG, Kranz DM (2000) IL-12 treatment of endogenously arising murine brain tumors. J Immunol 165:7293–7299
Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P et al (2009) Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325:612–616
Thongtan T, Cheepsunthorn P, Chaiworakul V, Rattanarungsan C, Wikan N, Smith DR (2009) Highly permissive infection of microglial cells by Japanese encephalitis virus: a possible role as a viral reservoir. Microbes Infect 12(1):37–45
Van Der Voorn P, Tekstra J, Beelen RH, Tensen CP, Van Der Valk P, De Groot CJ (1999) Expression of MCP-1 by reactive astrocytes in demyelinating multiple sclerosis lesions. Am J Pathol 154:45–51
Wilkins BS, Wright DH (2000) Illustrated Pathology of the spleen. Cambridge University Press, Cambridge
Yang KD, Yeh WT, Chen RF, Chuon HL, Tsai HP, Yao CW, Shaio MF (2004) A model to study neurotropism and persistency of Japanese encephalitis virus infection in human neuroblastoma cells and leukocytes. J Gen Virol 85:635–642
Acknowledgement
This work is supported by the grant awarded to A.B. from the Council of Scientific and Industrial Research (27(0173)/07/EMR-II), Government of India. K.D. is a recipient of the research associateship in the biotechnology and life sciences from the Department of Biotechnology, Government of India; A.N. is a recipient of the Junior Research Fellowship from the Council of Scientific and Industrial Research Government of India; and M.K.M was a recipient of the Senior Research Fellowship from the University Grants Commission, Government of India. We thank Manish Kumar Dogra for his technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dutta, K., Kumawat, K.L., Nazmi, A. et al. Minocycline Differentially Modulates Viral Infection and Persistence in an Experimental Model of Japanese Encephalitis. J Neuroimmune Pharmacol 5, 553–565 (2010). https://doi.org/10.1007/s11481-010-9233-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-010-9233-8