Abstract
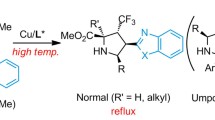
A copper-catalyzed highly stereoselective cyclopropanation of 1,2-disubstituted olefins with α-nitrodiazo acetates has been developed, giving the desired products in up to 97 % yields, up to >99/1 dr and up to 98 % ee, which provides an efficient access to the synthesis of optical active cyclopropane α-amino acids and unnatural α-amino acid derivatives.


Similar content being viewed by others
References
Brackmann F, de Meijere A (2007) Natural occurrence, syntheses, and applications of cyclopropyl-group-containing alpha-amino acids. 1. 1-Aminocyclopropanecarboxylic acid and other 2,3-methanoamino acids. Chem Rev 107:4493–4537
Brackmann F, de Meijere A (2007) Natural occurrence, syntheses, and applications of cyclopropyl-group-containing alpha-amino acids. 2. 3,4- and 4,5-Methanoamino acids. Chem Rev 107:4538–4583
Seebach D, Haner R, Vettiger T (1987) Nucleophilic ring-opening of aryl-α-nitrocyclopropanecarboxylates with sterically protected but electronically effective carbonyl and nitro-group—a new principle of α-amino-acid synthesis (2-aminobutanoic acid a4-synthon). Helv Chim Acta 70:1507–1515
Vettiger T, Seebach D (1990) Nucleophilic ring-opening of aryl 1-nitro-1-cyclopropanecarboxylate with sterically protected, but electronically effective carbonyl and nitro-group—a new principle of amino-acid synthesis. Liebigs Ann Chem 195–201
Charette AB, Wurz R (2003) Progress towards asymmetric intermolecular and intramolecular cyclopropanations using α-nitro-α-diazo carbonyl substrates. J Mol Catal A Chem 196:83–91
Lindsay VN, Lin W, Charette AB (2009) Experimental evidence for the all-up reactive conformation of chiral rhodium(II) carboxylate catalysts: enantioselective synthesis of cis-cyclopropane α-amino acids. J Am Chem Soc 131:16383–16385
Zhu S, Perman JA, Zhang XP (2008) Acceptor/acceptor-substituted diazo reagents for carbene transfers: cobalt-catalyzed asymmetric Z-cyclopropanation of alkenes with α-nitrodiazoacetates. Angew Chem Int Ed 47:8460–8463
Doyle MP, McKervey MA, Ye T (1998) Modern catalytic methods for organic synthesis with diazo compounds: from cyclopropanes to ylides. Wiley, New York
Davies HML, Antoulinakis EG (2001) Intermolecular metal-catalyzed carbenoid cyclopropanations. Organic reactions, vol 57. Wiley, New York, pp 1–326
Lebel H, Marcoux JF, Molinaro C et al (2003) Stereoselective cyclopropanation reactions. Chem Rev 103:977–1050
Roy MN, Lindsay VNG, Charette AB (2011) Stereoselective synthesis 1: stereoselective reactions of carbon–carbon double bonds. Georg Thieme-Verlag, New York
Davies HML, Bruzinski PR, Lake DH et al (1996) Asymmetric cyclopropanations by rhodium(II) N-(arylsulfonyl)prolinate catalyzed decomposition of vinyldiazomethanes in the presence of alkenes. Practical enantioselective synthesis of the four stereoisomers of 2-phenylcyclopropan-1-amino Acid. J Am Chem Soc 118:6897–6907
Denton JR, Cheng K, Davies HML (2008) Stereoselective construction of nitrile-substituted cyclopropanes. Chem Commun 10:1238–1240
Lin W, Charette AB (2005) Rhodium-catalyzed asymmetric intramolecular cyclopropanation of substituted allylic cyanodiazoacetates. Adv Synth Catal 347:1547–1552
Lindsay VN, Fiset D, Gritsch PJ et al (2013) Stereoselective Rh2(S-IBAZ)4-catalyzed cyclopropanation of alkenes, alkynes, and allenes: asymmetric synthesis of diacceptor cyclopropylphosphonates and alkylidenecyclopropanes. J Am Chem Soc 135:1463–1470
Lindsay VN, Nicolas C, Charette AB (2011) Asymmetric Rh(II)-catalyzed cyclopropanation of alkenes with diacceptor diazo compounds: p-methoxyphenyl ketone as a general stereoselectivity controlling group. J Am Chem Soc 133:8972–8981
Marcoux D, Azzi S, Charette AB (2009) TfNH2 as achiral hydrogen-bond donor additive to enhance the selectivity of a transition metal catalyzed reaction. Highly enantio- and diastereoselective rhodium-catalyzed cyclopropanation of alkenes using α-cyano diazoacetamide. J Am Chem Soc. 131:6970–6972
Marcoux D, Charette AB (2008) Trans-directing ability of amide groups in cyclopropanation: application to the asymmetric cyclopropanation of alkenes with diazo reagents bearing two carboxy groups. Angew Chem Int Ed 47:10155–10158
Marcoux D, Goudreau SR, Charette AB (2009) Trans-directing ability of the amide group: enabling the enantiocontrol in the synthesis of 1,1-dicarboxy cyclopropanes. Reaction development, scope, and synthetic applications. J Org Chem 74:8939–8955
Nishimura T, Maeda Y, Hayashi T (2010) Asymmetric cyclopropanation of alkenes with dimethyl diazomalonate catalyzed by chiral diene-rhodium complexes. Angew Chem Int Ed 49:7324–7327
Xu X, Lu H, Ruppel JV et al (2011) Highly asymmetric intramolecular cyclopropanation of acceptor-substituted diazoacetates by Co(II)-based metalloradical catalysis: iterative approach for development of new-generation catalysts. J Am Chem Soc 133:15292–15295
Xu X, Zhu S, Cui X et al (2013) Cobalt(II)-catalyzed asymmetric olefin cyclopropanation with α-ketodiazoacetates. Angew Chem Int Ed 52:11857–11861
Zhu S, Xu X, Perman JA et al (2010) A general and efficient cobalt(II)-based catalytic system for highly stereoselective cyclopropanation of alkenes with α-cyanodiazoacetates. J Am Chem Soc 132:12796–12799
Johansen MB, Kerr MA (2010) Direct functionalization of indoles: copper-catalyzed malonyl carbenoid insertions. Org Lett 12:4956–4959
Titanyuk ID, Beletskaya IP, Peregudov AS et al (2007) Trifluoromethylated cyclopropanes and epoxides from CuI-mediated transformations of α-trifluoromethyl-diazophosphonate. J Fluor Chem 128:723–728
Moreau B, Charette AB (2005) Expedient synthesis of cyclopropane α-amino acids by the catalytic asymmetric cyclopropanation of alkenes using iodonium ylides derived from methyl nitroacetate. J Am Chem Soc 127:18014–18015
Matveeva ED, Proskurnina MV, Zefirov NS (2006) Polyvalent iodine in organic chemistry: recent developments, 2002–2005. Heteroat Chem 17:595–617
Moreau B, Alberico D, Lindsay VNG et al (2012) Catalytic asymmetric synthesis of nitrocyclopropane carboxylates. Tetrahedron 68:3487–3496
Müller P (2004) Asymmetric transfer of carbenes with phenyliodonium ylides. Acc Chem Res 37:243–251
Long J, Yuan Y, Shi Y (2003) Asymmetric Simmons-Smith cyclopropanation of unfunctionalized olefins. J Am Chem Soc 125:13632–13633
Cao ZY, Wang XM, Tan C et al (2013) Highly stereoselective olefin cyclopropanation of diazooxindoles catalyzed by a C 2-symmetric spiroketal bisphosphine/Au(I) complex. J Am Chem Soc 135:8197–8200
Li J, Liao S, Xiong H et al (2012) Highly diastereo- and enantioselective cyclopropanation of 1,2-disubstituted alkenes. Angew Chem Int Ed 51:8838–8841
Xu ZH, Zhu SN, Sun XL et al (2007) Sidearm effects in the enantioselective cyclopropanation of alkenes with aryldiazoacetates catalyzed by trisoxazoline/Cu(I). Chem Commun 19:1960–1962
Desimoni G, Faita G, Jørgensen KA (2006) C 2-symmetric chiral bis(oxazoline) ligands in asymmetric catalysis. Chem Rev 106:3561–3651
Hargaden GC, Guiry PJ (2009) Recent applications of oxazoline-containing ligands in asymmetric catalysis. Chem. Rev 109:2505–2550
Johnson JS, Evans DA (2000) Chiral bis(oxazoline) copper(II) complexes: versatile catalysts for enantioselective cycloaddition, aldol, Michael, and carbonyl ene reactions. Acc Chem Res 33:325–335
Jørgensen KA, Johannsen M, Yao SL et al (1999) Catalytic asymmetric addition reactions of carbonyls. A common catalytic approach. Acc Chem Res 32:605–613
McManus HA, Guiry PJ (2004) Recent developments in the application of oxazoline-containing ligands in asymmetric catalysis. Chem Rev 104:4151–4202
Pfaltz A (1993) Chiral semicorrins and related nitrogen-heterocycles as ligands in asymmetric catalysis. Acc Chem Res 26:339–345
Gade LH, Bellemin-Laponnaz S (2008) Exploiting threefold symmetry in asymmetric catalysis: the case of tris(oxazolinyl)ethanes (“trisox”). Chem Eur J 14:4142–4152
Hargaden GC, Guiry PJ (2009) Recent applications of oxazoline-containing ligands in asymmetric catalysis. Chem Rev 109:2505–2550
Liao SH, Sun XL, Tang Y (2014) Side arm strategy for catalyst design: modifying bisoxazolines for remote control of enantioselection and related. Acc Chem Res 47:2260–2272
Zhou J, Tang Y (2005) The development and application of chiral trisoxazolines in asymmetric catalysis and molecular recognition. Chem Soc Rev 34:664–676
Deng C, Wang LJ, Zhu J et al (2012) A chiral cagelike copper(I) catalyst for the highly enantioselective synthesis of 1,1-cyclopropane diesters. Angew Chem Int Ed 51:11620–11623
Xiong H, Xu H, Liao S et al (2013) Copper-catalyzed highly enantioselective cyclopentannulation of indoles with donor-acceptor cyclopropanes. J Am Chem Soc 135:7851–7854
Zhu SF, Zhou QL (2012) Transition-metal-catalyzed enantioselective heteroatom-hydrogen bond insertion reactions. Acc Chem Res 45:1365–1377
Li W, Liu XH, Hao XY et al (2011) New electrophilic addition of α-diazoesters with ketones for enantioselective C–N bond formation. J Am Chem Soc 133:15268–15271
Li W, Liu XH, Tan F et al (2013) Catalytic asymmetric homologation of α-ketoesters with α-diazoesters: synthesis of succinate derivatives with chiral quaternary centers. Angew Chem Int Ed 52:10883–10886
Zhu Y, Liu XH, Dong SX et al (2014) Asymmetric N–H insertion of secondary and primary anilines under the catalysis of palladium and chiral guanidine derivatives. Angew Chem Int Ed 53:1636–1640
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21421091, 21432011 and 21272250) and the Chinese Academy of Sciences.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Liang-Wen Feng and Peng Wang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Feng, LW., Wang, P., Wang, L. et al. Copper(I)/SaBOX catalyzed highly diastereo- and enantio-selective cyclopropanation of cis-1,2-disubstituted olefins with α-nitrodiazoacetates. Sci. Bull. 60, 210–215 (2015). https://doi.org/10.1007/s11434-014-0708-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-014-0708-5