Abstract
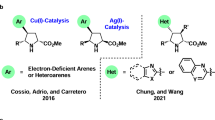
Copper-catalyzed asymmetric 1,3-dipolar cycloaddition of azomethine ylides and β-trifluoromethyl-substituted alkenyl heteroarenes was developed for the first time. A wide range of enantioenriched pyrrolidines containing both heteroarenes and trifluoromethyl group with multiple stereogenic centers could be readily accessible by this method with good to high yields and excellent levels of both stereo- and regioselectivity (up to 99% yield, >20:1 rr, >20:1 dr, and up to 95% ee). Notably, substrate-controlled umpolung-type dipolar cycloaddition was also disclosed in this protocol to achieve regiodivergent synthesis with α-aryl substituted aldimine esters as the dipole precursors. Systematic DFT studies were conducted to explore the origin of the stereo- and regioselectivity of this 1,3-dipolar cycloaddition, and suggest that copper(II) salt utilized in this catalytic system could be reduced in-situ to the active copper(I) species and might be responsible for the observed high stereo- and regioselectivity.

Similar content being viewed by others
References
Enders D, Thiebes C. Pure Appl Chem, 2001, 73: 573–578
Harwood LM, Vickers RJ. in Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products. Padwa A and Pearson W, eds. Hoboken: John Wiley & Sons, Ltd., 2002
Pyne S, Davis A, Gates N, Hartley J, Lindsay K, Machan T, Tang M. Synlett, 2004, 2004: 2670–2680
Michael JP. Nat Prod Rep, 2008, 25: 139–165
Mukaiyama T, Asami M. Top Curr Chem, 1985, 127: 133–167
Vicario JL, Badia D, Carrillo L, Ruiz N, Reyes E. Targets Heterocyclic Syst, 2008, 12: 302–327
Bhat C, Tilve SG. RSC Adv, 2014, 4: 5405–5452
Sulzer-Mossé S, Alexakis A. Chem Commun, 2007, 30: 3123–3135
Jensen KL, Dickmeiss G, Jiang H, Albrecht Ł, Jørgensen KA. Acc Chem Res, 2012, 45: 248–264
Li X, Li J. Mini-Rev Med Chem, 2010, 10: 794–805
Vega-Peñaloza A, Paria S, Bonchio M, Dell’Amico L, Companyó X. ACS Catal, 2019, 9: 6058–6072
Cossy J, Pardo DG. Targets Heterocyclic Syst, 2002, 6: 1–26
Chelucci G, Murineddu G, Pinna GA. Tetrahedron-Asymmetry, 2004, 15: 1373–1389
Liu X, Lin L, Feng X. Org Chem Front, 2014, 1: 298–302
Daly JW. J Med Chem, 2003, 46: 445–452
Harrity JPA, Provoost O. Org Biomol Chem, 2005, 3: 1349–1358
Escolano C, Amat M, Bosch J. Chem Eur J, 2006, 12: 8198–8207
Vitaku E, Smith DT, Njardarson JT. J Med Chem, 2014, 57: 10257–10274
Jiang W, Li Y, Wang Z. Chem Soc Rev, 2013, 42: 6113–6127
Kukhar VP and Soloshonok VA, ed. Fluorine Containing Amino Acids—Synthesis and Properties. Chichester: Wiley, 1995
Shimizu M, Hiyama T. Angew Chem Int Ed, 2005, 44: 214–231
Schlosser M. Angew Chem Int Ed, 2006, 45: 5432–5446
Uneyama K, Katagiri T, Amii H. Acc Chem Res, 2008, 41: 817–829
Smits R, Cadicamo CD, Burger K, Koksch B. Chem Soc Rev, 2008, 37: 1727–1739
Nie J, Guo HC, Cahard D, Ma JA. Chem Rev, 2010, 111: 455–529
Merino E, Nevado C. Chem Soc Rev, 2014, 43: 6598–6608
Ojima I, Macarthy JR, Welch JT. Biomedical Frontiers of Fluorine Chemistry. New York: American Chemical Society, 1996
Luzina EL, Popov AV. J Fluorine Chem, 2014, 168: 121–127
Kaur K, Kumar V, Gupta GK. J Fluorine Chem, 2015, 178: 306–326
Zanda M. New J Chem, 2004, 28: 1401–1411
Dmowski W. Wiadomosci Chemiczne, 1997, 51: 263–291
Tredwell M, Gouverneur V. Edited by Carreira EM, Yamamoto H. Fluorine in medicinal chemistry: Importance of chirality. Compre Chiral, 2012, 1: 70–85
Huisgen R. Angew Chem Int Ed, 1963, 2: 565–598
Padwa A and Pearson WH. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products. New York: Wiley-VCH, 2002
Hashimoto T, Maruoka K. Chem Rev, 2015, 115: 5366–5412
Adrio J, Carretero JC. Chem Commun, 2019, 55: 11979–11991
Wei L, Chang X, Wang CJ. Acc Chem Res, 2020, 53: 1084–1100
Zhao P, Li Z, He J, Liu X, Feng X. Sci China Chem, 2021, 64: 1355–1360
Li YN, Chang X, Xiong Q, Dong XQ, Wang CJ. Chin Chem Lett, 2021, 32: 4029–4032
Stohler R, Wahl F, Pfaltz A. Synthesis, 2005, 2005: 1431–1436
Chen XH, Wei Q, Luo SW, Xiao H, Gong LZ. J Am Chem Soc, 2009, 131: 13819–13825
Cristóbal C, Gaviña D, Alonso I, Ribagorda M, Carretero JC, del Pozo C, Adrio J. Chem Commun, 2022, 58: 7805–7808
Deng Y, Dong Z, Gao F, Guo Y, Sun M, Li Y, Wang Y, Chen Q, Wang K, Yan W. J Org Chem, 2021, 86: 13011–13024
Feng B, Lu LQ, Chen JR, Feng G, He BQ, Lu B, Xiao WJ. Angew Chem Int Ed, 2018, 57: 5888–5892
Shen C, Yang Y, Wei L, Dong WW, Chung LW, Wang CJ. iScience, 2019, 11: 146–159
Xu S, Zhang ZM, Xu B, Liu B, Liu Y, Zhang J. J Am Chem Soc, 2018, 140: 2272–2283
Gill M, Das A, Singh VK. Org Lett, 2022, 24: 5629–5634
Li QH, Tong MC, Li J, Tao HY, Wang CJ. Chem Commun, 2011, 47: 11110–11112
Li QH, Xue ZY, Tao HY, Wang CJ. Tetrahedron Lett, 2012, 53: 3650–3653
López-Pérez A, Adrio J, Carretero J. Angew Chem Int Ed, 2009, 48: 340–343
Tong MC, Li J, Tao HY, Li YX, Wang CJ. Chem Eur J, 2011, 17: 12922–12927
Chang X, Yang Y, Shen C, Xue KS, Wang ZF, Cong H, Tao HY, Chung LW, Wang CJ. J Am Chem Soc, 2021, 143: 3519–3535
See computational details in the Supplementary Information
Lam Y, Grayson MN, Holland MC, Simon A, Houk KN. Acc Chem Res, 2016, 49: 750–762
Peng Q, Paton RS. Acc Chem Res, 2016, 49: 1042–1051
Tantillo DJ. Acc Chem Res, 2016, 49: 741–749
Ahn S, Hong M, Sundararajan M, Ess DH, Baik MH. Chem Rev, 2019, 119: 6509–6560
Harvey JN, Himo F, Maseras F, Perrin L. ACS Catal, 2019, 9: 6803–6813
Lan J, Li X, Yang Y, Zhang X, Chung LW. Acc Chem Res, 2022, 55: 1109–1123
Ess DH, Houk KN. J Am Chem Soc, 2008, 130: 10187–10198
Wang M, Wang CJ, Lin Z. Organometallics, 2012, 31: 7870–7876
Pascual-Escudero A, de Cózar A, Cossío FP, Adrio J, Carretero JC. Angew Chem Int Ed, 2016, 55: 15334–15338
Domingo LR, Ríos-Gutiérrez M, Pérez P. J Org Chem, 2018, 83: 10959–10973
Cheng F, Kalita SJ, Zhao Z, Yang X, Zhao Y, Schneider U, Shibata N, Huang Y. Angew Chem Int Ed, 2019, 58: 16637–16643
Xiong Y, Du Z, Chen H, Yang Z, Tan Q, Zhang C, Zhu L, Lan Y, Zhang M. J Am Chem Soc, 2019, 141: 961–971
Li B, Xu H, Dang Y, Houk KN. J Am Chem Soc, 2022, 144: 1971–1985
Chang X, Liu XT, Li F, Yang Y, Chung LW, Wang CJ. Chem Sci, 2023, 14: 5460–5469
Xu L, Chung LW, Wu YD. ACS Catal, 2016, 6: 483–493
Gao W, Lv H, Zhang T, Yang Y, Chung LW, Wu YD, Zhang X. Chem Sci, 2017, 8: 6419–6422
Lan J, Liao T, Zhang T, Chung LW. Inorg Chem, 2017, 56: 6809–6819
Zhang X, Chung LW. Chem Eur J, 2017, 23: 3623–3630
Wu SB, Zhang T, Chung LW, Wu YD. Org Lett, 2019, 21: 360–364
Yang Y, Zhang X, Zhong LP, Lan J, Li X, Li CC, Chung LW. Nat Commun, 2020, 11: 1850
Du X, Xiao Y, Yang Y, Duan Y, Li F, Hu Q, Chung LW, Chen G, Zhang X. Angew Chem Int Ed, 2021, 60: 11384–11390
Lan J, Zhang T, Yang Y, Li X, Chung LW. Inorg Chem, 2022, 61: 18019–18032
Morokuma K. Acc Chem Res, 1977, 10: 294–300
Ess DH, Houk KN. J Am Chem Soc, 2007, 129: 10646–10647
Bickelhaupt FM, Houk KN. Angew Chem Int Ed, 2017, 56: 10070–10086
Chen C, Zhang Z, Jin S, Fan X, Geng M, Zhou Y, Wen S, Wang X, Chung LW, Dong XQ, Zhang X. Angew Chem Int Ed, 2017, 56: 6808–6812
Kalita SJ, Zhao Z, Li Z, Cheng F, Zhao Y, Huang Y. Eur J Org Chem, 2021, 2021(40): 5530–5535
Calvo JS, Villones RLE, York NJ, Stefaniak E, Hamilton GE, Stelling AL, Bal W, Pierce BS, Meloni G. J Am Chem Soc, 2022, 144: 709–722
Mooibroek TJ, Aromí G, Quesada M, Roubeau O, Gamez P, DeBeer George S, van Slageren J, Yasin S, Ruiz E, Reedijk J. Inorg Chem, 2009, 48: 10643–10651
Castañeda-Arriaga R, Pérez-González A, Alvarez-Idaboy JR, Galano A. Int J Quantum Chem, 2018, 118: e25527
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22071186, 22071187, 22073067, 22101216, 22271226, 21933003, 22193020, 22193023), the National Youth Talent Support Program, the Natural Science Foundation of Hubei Province (2020CFA036 2021CFA069), the Fundamental Research Funds for the Central Universities (2042022kf1180, 2042022kf1040), the Shenzhen Nobel Prize Scientists Laboratory Project (C17783101) and the Guangdong Provincial Key Laboratory of Catalytic Chemistry (2020B121201002). We thank the Center for Computational Science and Engineering at the Southern University of Science and Technology and CHEM HPC at SUSTech for partly supporting this work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information
The supporting information is available online at chem.scichina.com and link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supporting Information
11426_2023_1683_MOESM1_ESM.pdf
Copper-catalyzed asymmetric 1,3-dipolar cycloaddition of azomethine ylides with β-trifluoromethyl-substituted alkenyl heteroarenes
Rights and permissions
About this article
Cite this article
Cheng, X., Chang, X., Yang, Y. et al. Copper-catalyzed asymmetric 1,3-dipolar cycloaddition of azomethine ylides with β-trifluoromethyl-substituted alkenyl heteroarenes. Sci. China Chem. 66, 3193–3204 (2023). https://doi.org/10.1007/s11426-023-1683-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-023-1683-9