Abstract
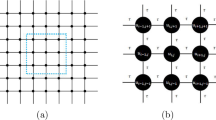
Spiral waves have been observed in the biological experiments on rat cortex perfused with drugs which can block inhibitory synapse and switch neuron excitability from type II to type I. To simulate the spiral waves observed in the experiment, the spatiotemporal patterns are investigated in a network composed of neurons with type I and II excitabilities and excitatory coupling. Spiral waves emerge when the percentage (p) of neurons with type I excitability in the network is at middle levels, which is dependent on the coupling strength. Compared with other spatial patterns which appear at different p values, spiral waves exhibit optimal spatial correlation at a certain spatial frequency, implying the occurrence of spatial coherence resonance-like phenomenon. Some dynamical characteristics of the network such as mean firing frequency and synchronous degree can be well interpreted with distinct properties between type I excitability and type II excitability. The results not only identify dynamics of spiral waves in neuronal networks composed of neurons with different excitabilities, but also are helpful to understanding the emergence of spiral waves observed in the biological experiment.
Similar content being viewed by others
References
Gorelova N A, Bureš J. Spiral waves of spreading depression in the isolated chicken retina. J Neurobiol, 1983, 14: 353–363
Salomonsz R, Pertsov A V, Davidenko J M, et al. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature, 1992, 355: 349–351
Prechtl J C, Cohen L B, Pesaran B, et al. Visual stimuli induce waves of electrical activity in turtle cortex. Proc Natl Acad Sci U S A, 1997, 94: 7621–7626
Engel A K, König P, Kreiter A K, et al. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science, 1991, 252: 1177–1179
Cobb S R, Buhl E H, Halasy K, et al. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature, 1995, 378: 75–78
Uhlhaas P J, Roux F, Rodriguez E, et al. Neural synchrony and the development of cortical networks. Trends Cogn Sci, 2010, 14: 72–80
Huang X Y, Troy W C, Yang Q, et al. Spiral waves in disinhibited mammalian neocortex. J Neurosci, 2004, 24: 9897–9902
Schiff S J, Huang X Y, Wu J Y. Dynamical evolution of spatiotemporal patterns in mammalian middle cortex. Phys Rev Lett, 2007, 98: 178102
Huang X Y, Xu W F, Liang J M, et al. Spiral wave dynamics in neocortex. Neuron, 2010, 68: 978–990
Jalife J. Rotors and spiral waves in atrial fibrillation. J Cardiovasc Electrophysiol, 2003, 14: 776–780
Stiefel K M, Gutkin B S, Sejnowski T J. Cholinergic neuromodulation changes phase response curve shape and type in cortical pyramidal neurons. PLoS One, 2008, 3: e3947
Perc M. Spatial coherence resonance in excitable media. Phys Rev E, 2005, 72: 016207
Liu Z Q, Zhang H M, Li Y Y, et al. Multiple spatial coherence resonance induced by the stochastic signal in neuronal networks near a saddle-node bifurcation. Physica A, 2010, 389: 2642–2653
Sun X J, Lu Q S. Spatial coherence resonance induced by colored noise and parameter diversity in a neuronal network. Chin Phys B, 2010, 19: 040504
Tang Z, Li Y Y, Xi L, et al. Spiral waves and multiple spatial coherence resonances induced by colored noise in neuronal network. Commun Theor Phys, 2012, 57: 61–67
Gu H G, Jia B, Li Y Y, et al. White noise-induced spiral waves and multiple spatial coherence resonances in a neuronal network with type I excitability. Physica A, 2013, 392: 1361–1374
Wu Y, Li J J, Liu S B, et al. Noise-induced spatiotemporal patterns in Hodgkin–Huxley neuronal network. Cogn Neurodyn, 2013, 7: 431–440
Perc M. Spatial decoherence induced by small-world connectivity in excitable media. New J Phys, 2005, 7: 252
Sun X J, Perc M, Lu Q S, et al. Spatial coherence resonance on diffusive and small-world networks of Hodgkin—Huxley neurons. Chaos, 2008, 18: 023102
Glatt E, Gassel M, Kaiser F. Variability-sustained pattern formation in sub-excitable media. Phys Rev E, 2007, 75: 026206
Tang J, Yang L, Ma J, et al. Ca2+ spiral waves in a spatially discrete and random medium. Eur Biophys J Biophys Lett, 2009, 38: 1061–1068
Tang J, Yi M, Chen P, et al. The influence of diversity on spiral wave in the cardiac tissue. Europhys Lett, 2012, 97: 28003
Li Y Y, Jia B, Gu H G, et al. Parameter diversity induced multiple spatial coherence resonances and spiral waves in neuronal network with and without noise. Commun Theor Phys, 2012, 57: 817–824
Qin H X, Ma J, Wang C N, et al. Autapse-induced target wave, spiral wave in regular network of neurons. Sci China-Phys Mech Astron, 2014, 57: 1918–1926
Qin H X, Ma J, Jin W Y, et al. Dynamics of electric activities in neuron and neurons of network induced by autapses. Sci China Tech Sci, 2014, 57: 936–946
Qin H X, Ma J, Wang C N, et al. Autapse-induced spiral wave in network of neurons under noise. PloS One, 2014, 9: e100849
Ma J, Song X, Tang J, et al. Wave emitting and propagation induced by autapse in a forward feedback neuronal network. Neurocomputing, 2015, 167: 378–389
Song X L, Wang C N, Ma J, et al. Transition of electric activity of neurons induced by chemical and electric autapses. Sci China Tech Sci, 2015, 58: 1007–1014
Liu S B, Wu Y, Li J J, et al. The dynamic behavior of spiral waves in stochastic Hodgkin–Huxley neuronal networks with ion channel blocks. Nonlinear Dyn, 2013, 73: 1055–1063
Sun X J, Shi X. Effects of channel blocks on the spiking regularity in clustered neuronal networks. Sci China Tech Sci, 2014, 57: 879–884
Li Y Y, Gu H G. The influence of initial values on spatial coherence resonance in neuronal networks. Int J Bifurcat Chaos, 2015, 25: 1550104
Hodgkin A L. The local electric changes associated with repetitive action in a non-medullated axon. J Physiol, 1948, 107: 165–181
Rinzel J, Ermentrout G B. Analysis of neural excitability and oscillations. In: Methods in Neural Modeling. Cambridge: The MIT Press, 1989. 135–171
Izhikevich E M. Neural excitability, spiking and bursting. Int J Bifurcat Chaos, 2000, 10: 1171–1266
Hansel D, Mato G, Meunier C. Synchrony in excitatory neural networks. Neural Comput, 1995, 7: 307–337
Tateno T, Harsch A, Robinson H P C. Threshold firing frequency- current relationships of neurons in rat somatosensory cortex: Type 1 and type 2 dynamics. J Neurophysiol, 2004, 92: 2283–2294
Tateno T, Robinson H P C. Rate coding and spike-time variability in cortical neurons with two types of threshold dynamics. J Neurophysiol, 2006, 95: 2650–2663
Tsubo Y, Takada M, Reyes A D, et al. Layer and frequency dependencies of phase response properties of pyramidal neurons in rat motor cortex. Eur J Neurosci, 2007, 25: 3429–3441
Jia B, Gu H G, Li Y Y. Coherence-resonance-induced neuronal firing near a saddle-node and homoclinic bifurcation corresponding to type-I excitability. Chin Phys Lett, 2011, 28: 090507
Jia B, Gu H G. Identifying type I excitability using dynamics of stochastic neural firing patterns. Cogn Neurodyn, 2012, 6: 485–497
Prescott S A, Ratté S, Koninck Y D, et al. Pyramidal neurons switch from integrators in vitro to resonators under in vivo-like conditions. J Neurophysiol, 2008, 100: 3030–3042
Gu H G, Chen S G. Potassium-induced bifurcations and chaos of firing patterns observed from biological experiment on a neural pacemaker. Sci China Tech Sci, 2014, 57: 864–871
Gu H G, Zhao Z G. Dynamics of time delay-induced multiple synchronous behaviors in inhibitory coupled bursting neurons. PLoS One, 2015, 10: e0138593
Ermentrout G B. Type I membranes, phase resetting curves, and synchrony. Neural Comput, 1996, 8: 979–1001
Galán R F, Bard Ermentrout G, Urban N N. Reliability and stochastic synchronization in type I vs type II neural oscillators. Neurocomputing, 2007, 70: 2102–2106
Marella S, Ermentrout G. Class-II neurons display a higher degree of stochastic synchronization than class-I neurons. Phys Rev E, 2008, 77: 041918
Aushra A, Ermentrout G B. Type-II phase resetting curve is optimal for stochastic synchrony. Phys Rev E, 2009, 80: 011911
Bogaard A, Parent J, Zochowski M, et al. Interaction of cellular and network mechanisms in spatiotemporal pattern formation in neuronal networks. J Neurosci, 2009, 29: 1677–1687
Smeal R M, Ermentrout G B, White J A. Phase-response curves and synchronized neural networks. Philos T Roy Soc B, 2010, 365: 2407–2422
Jiao X F, Zhu D F. Phase-response synchronization in neuronal population. Sci China Tech Sci, 2014, 57: 923–928
Morris C, Lecar H. Voltage oscillations in the barnacle giant muscle fiber. Biophys J, 1981, 35: 193–213
Tateno T, Pakdaman K. Random dynamics of the Morris-Lecar neural model. Chaos, 2004, 14: 511–530
Tsumoto K, Kitajima H, Yoshinaga T, et al. Bifurcations in Morris- Lecar neuron model. Neurocomputing, 2006, 69: 293–316
Shen Y, Hou Z, Xin H. Transition to burst synchronization in coupled neuron networks. Phys Rev E, 2008, 77: 03192
Gutkin B S, Ermentrout G B. Dynamics of membrane excitability determine inter-spike interval variability: A link between spike generation mechanisms and cortical spike train statistics. Neural Comput, 1998, 10: 1047–1065
Feng J F, Brown D. Coefficient of variation of interspike intervals greater than 0.5. How and when? Biol Cybern, 1999, 80: 291–297
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, W., Gu, H. & Liu, M. Spatiotemporal dynamics in a network composed of neurons with different excitabilities and excitatory coupling. Sci. China Technol. Sci. 59, 1943–1952 (2016). https://doi.org/10.1007/s11431-016-6046-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-016-6046-x