Abstract
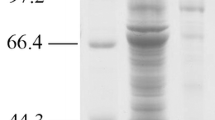
A recombinant gene expressing a Cry1Ac-GFP fusion protein with a molecular mass of approximately 160 kD was constructed to investigate the expression of cry1Ac, the localization of its gene product Cry1Ac, and its role in crystal development in Bacillus thuringiensis. The cry1Ac-gfp fusion gene under the control of the cry1Ac promoter was cloned into the plasmid pHT304, and this construct was designated pHTcry1Ac-gfp. pHTcry1Ac-gfp was transformed into the crystal-negative strain, HD-73 cry−, and the resulting strain was named HD-73−(pHTcry1Ac-gfp). The gfp gene was then inserted into the large HD-73 endogenous plasmid pHT73 and fused with the 3′ terminal of the cry1Ac gene by homologous recombination, yielding HD-73Φ(cry1Ac-gfp)3534. Laser confocal microscopy and Western blot analyses showed for the first time that the Cry1Ac-GFP fusion proteins in both HD-73−(pHTcry1Ac-gfp) and HD-73Φ(cry1Ac-gfp)3534 were produced during asymmetric septum formation. Surprisingly, the Cry1Ac-GFP fusion protein showed polarity and was located near the septa in both strains. There was no significant difference between Cry1Ac-GFP and Cry1Ac in their toxicity to Plutella xylostella larvae.
Similar content being viewed by others
References
Schnepf E, Crickmore N, Van R J, et al. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev, 1998, 62:775–806 1:CAS:528:DyaK1cXmtFOju7w%3D, 9729609
Agaisse H, Lereclus D. How does Bacillus thuringiensis produce so much insecticidal crystal protein? J Bacteriol, 1995, 177:6027–6032 1:CAS:528:DyaK2MXptVequrc%3D, 7592363
Agaisse H, Lereclus D. Structural and functional analysis of the promoter region involved in full expression of the cryIIIA toxin gene of Bacillus thuringiensis. Mol Microbiol, 1994, 13:97–107 1:CAS:528:DyaK2cXlt1KltLw%3D, 10.1111/j.1365-2958.1994.tb00405.x, 7984098
Oestergaard J, Ehlers R U, Martinez-Ramirez A C, et al. Binding of Cyt1Aa and Cry11Aa toxins of Bacillus thuringiensis serovar israelensis to brush border membrane vesicles of Tipula paludosa (Diptera: Nematocera) and subsequent pore formation. Appl Environ Microbiol, 2007, 73:3623–3629 1:CAS:528:DC%2BD2sXmtlKktLc%3D, 10.1128/AEM.01056-06, 17416690
Piggot P J, Hilbert D W. Sporulation of Bacillus subtilis. Curr Opin Microbiol, 2004, 7:579–586 1:CAS:528:DC%2BD2cXhtVWjtrvI, 10.1016/j.mib.2004.10.001, 15556029
Liu C W, Lin C C, Yiu J C, et al. Expression of a Bacillus thuringiensis toxin (cry1Ab) gene in cabbage (Brassica oleracea L. var. capitata L.) chloroplasts confers high insecticidal efficacy against Plutella xylostella. Theor Appl Genet, 2008, 117:75–88 1:CAS:528:DC%2BD1cXmtlWitrk%3D, 10.1007/s00122-008-0754-y, 18415072
Grover D, Yang J, Tavare S, et al. Simultaneous tracking of fly movement and gene expression using GFP. BMC Biotechnol, 2008, 8:93 10.1186/1472-6750-8-93, 19087237
Chelur D S, Ernstrom G G, Goodman M B, et al. The mechanosensory protein MEC-6 is a subunit of the C. elegans touch-cell degenerin channel. Nature, 2002, 420:669–673 1:CAS:528:DC%2BD38XpsVSitbg%3D, 10.1038/nature01205, 12478294
Ellermeier C D, Hobbs E C, Gonzalez-Pastor J E, et al. A three-protein signaling pathway governing immunity to a bacterial cannibalism toxin. Cell, 2006, 124:549–559 1:CAS:528:DC%2BD28Xhslaqs7g%3D, 10.1016/j.cell.2005.11.041, 16469701
Hahn J, Maier B, Haijema B J, et al. Transformation proteins and DNA uptake localize to the cell poles in Bacillus subtilis. Cell, 2005, 122:59–71 1:CAS:528:DC%2BD2MXmsFeitbY%3D, 10.1016/j.cell.2005.04.035, 16009133
Roh J Y, Li M S, Chang J H, et al. Expression and characterization of a recombinant Cry1Ac crystal protein with enhanced green fluorescent protein in acrystalliferous Bacillus thuringiensis. Lett Appl Microbiol, 2004, 38:393–399 1:CAS:528:DC%2BD2cXks1Grs7s%3D, 10.1111/j.1472-765X.2004.01505.x, 15059210
Roh J Y, Lee I H, Li M S, et al. Expression of a recombinant Cry1Ac crystal protein fused with a green fluorescent protein in Bacillus thuringiensis subsp. kurstaki Cry-B. J Microbiol, 2004, 42:340–345 1:CAS:528:DC%2BD2MXhtFaltLg%3D, 15650692
Bravo A, Agaisse H, Salamitou S, et al. Analysis of cryIAa expression in sigE and sigK mutants of Bacillus thuringiensis. Mol Gen Genet, 1996, 250:734–741 1:CAS:528:DyaK28XislOqtr4%3D, 8628234
Lereclus D, Arantes O, Chaufaux J, et al. Transformation and expression of a cloned delta-endotoxin gene in Bacillus thuringiensis. FEMS Microbiol Lett, 1989, 51:211–217 1:STN:280:DyaL1MznvVOiug%3D%3D, 2550317
Schaeffer P, Millet J, Aubert J P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA, 1965, 54:701–711 10.1073/pnas.54.3.704
Cormack B P, Valdivia R H, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP). Gene, 1996, 173:33–38 1:CAS:528:DyaK28XktVOlu7c%3D, 10.1016/0378-1119(95)00685-0, 8707053
Arantes O, Lereclus D. Construction of cloning vectors for Bacillus thuringiensis. Gene, 1991, 108:115–119 1:CAS:528:DyaK38Xlt1GktA%3D%3D, 10.1016/0378-1119(91)90495-W, 1662180
Arnaud M, Chastanet A, Débarbouillé M. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl Environ Microbiol, 2004, 70:6887–6891 1:CAS:528:DC%2BD2cXhtVSju7rF, 10.1128/AEM.70.11.6887-6891.2004, 15528558
Choma C T, Surewicz W K, Carey P R, et al. Secondary structure of the entomocidal toxin from Bacillus thuringiensis subsp. kurstaki HD-73. J Protein Chem, 1990, 9:87–94 1:CAS:528:DyaK3cXktlKlsLk%3D, 10.1007/BF01024989, 2340079
Mohan M, Gujar G T. Characterization and comparison of midgut proteases of Bacillus thuringiensis susceptible and resistant diamondback moth (Plutellidae: Lepidoptera). J Invertebr Pathol, 2003, 82:1–11 1:CAS:528:DC%2BD3sXhtVCjtbk%3D, 10.1016/S0022-2011(02)00194-5, 12581714
Espinasse S, Gohar M, Lereclus D, et al. An extracytoplasmic-function sigma factor is involved in a pathway controlling beta-exotoxin I production in Bacillus thuringiensis subsp. thuringiensis strain 407-1. J Bacteriol, 2004, 186:3108–3116 1:CAS:528:DC%2BD2cXktVGhu78%3D, 10.1128/JB.186.10.3108-3116.2004, 15126472
Mignot T, Mock M, Fouet A. A plasmid-encoded regulator couples the synthesis of toxins and surface structures in Bacillus anthracis. Mol Microbiol, 2003, 47:917–927 1:CAS:528:DC%2BD3sXhtlOmtrw%3D, 10.1046/j.1365-2958.2003.03345.x, 12581349
Bechtel D B, Bulla L A Jr. Electron microscope study of sporulation and parasporal crystal formation in Bacillus thuringiensis. J Bacteriol, 1976, 127:1472–1481 1:STN:280:DyaE283mtVSntQ%3D%3D, 182671
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yang, H., Rong, R., Song, F. et al. In vivo fluorescence observation of parasporal inclusion formation in Bacillus thuringiensis. Sci. China Life Sci. 53, 1106–1111 (2010). https://doi.org/10.1007/s11427-010-4058-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-010-4058-5