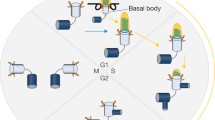
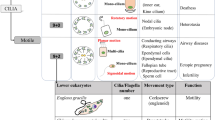
Abstract
This article looks mostly at the steps that have led to the primary cilium finding its place in our understanding of cell biology, developmental biology, and medical syndromes due to its aberrations. It is a personal account that stresses, if nothing else, the value of the adage “stick to your guns”. My obsession with this organelle, following on from fascination with the centriole, has led to a whole career devoted to determining the nature and role of primary cilia in basic cell biology, which has proved much more important than had been appreciated for almost a century. They are heavily involved in very many aspects of cell physiology that have much wider implications with regard to human biology and probably throughout the animal kingdom. That aberrations, to the surprise of many researchers in their structure or functioning has led to their being implicated or perhaps deeply involved in an extraordinary range of medical conditions. This invitation allows me to raise crucial questions that need answers regarding the regulation of their genesis, their cache of both intracellular and extracellular signal, and their association with a multitude of development processes from embryo to adult status.


Similar content being viewed by others
References
Agbu SO, Liang Y, Liu A, Anderson KV (2017) The small GTDase RSG1 controls a final step in primary cilia initiation. J Cell Biol. https://doi.org/10.1083/jcb.201604048
Bernabé-Rubio M, Alonso MA (2017) Routes and machinery of primary cilium biogenesis. Cell Mol Life Sci 74:30201–30206
Bloodgood RA (2009) From central to rudimentary to primary: the history of an under appreciated organelle whose time has come. The primary cilium. Methods Cell Biol 94:3–52. https://doi.org/10.1016/S0091-679X(08)94001-2
Currie AR, Wheatley DN (1966) Cilia of a distinctive structure (9+0) in endocrine and other tissues. Postgrad Med J 42:403–408
Dahl HA (1963) Fine structure of cilia in rat cerebral cortex. Z Zellforsch Mikrosk Anat 60:369–386
Gadadhar S, Dadi H, Bodakuntla S, Schnitzler A, Bieche I, Ruscin F, Janke C (2017) Tubulin glycylation controls primary cilia length. J Cell Biol 216:2701–2713
Goto H, Inoko A, Inagaki M (2013) Cell cycle progression by the repression of primary cilia formation in proliferating cells. Cell Mol Life Sci 70:3893–3905
Hampl M, Cela P, Szabo-Rogers HL, Bosakova MK, Dosedelova H, Krejci P (2017) Role of primary cilia in odontogenesis. J Dent Res 96:965–974
Kobayashi T, Itoh H (2017) Loss of a primary cilium in PDAC. Cell Cycle 16:817–818
Légaré S, Chabot C, Basik M (2017) SPEN, a new player in primary cilium formation and cell migration in breast cancer. Breast Cancer Res 19:104
Lepanto P, Badano JL, Zolessi FR (2016) Neuron’s little helper: The role of primary cilia in neurogenesis. Neurogenesis 3:e1248206. https://doi.org/10.1083/23262133.2016.1253363
Mazia D (1978) Origin of twoness in cell reproduction. In: Dirksen et al. (eds) Cell Reproduction – In Honor of Dan Mazia, Academic press, N Y, pps 1-14
McMurray RJ, Wann AK, Thompson CL, Connelly JT, Knight MM (2013) Surface topography regulates wnt signaling through control of primary cilia structure in mesenchymal stem cells. Sci Rep 3:3545
Moore ER, Jacobs CR (2017) The primary cilium as a signalling nexus for growth plate function and subsequent skeletal development. J Orthop Res. https://doi.org/10.1002/jor.23732
Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y et al (1998) Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95:829–837
Ong AC, Wheatley DN (2003) Polycystic kidney disease - the ciliary connection. Lancet 361:774–776
Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG (2000) Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol 151:709–718
Pitaval A, Senger F, Letort G, Gidrol X, Guyon L, Sillibourne J, Théry M (2017) Microtubule stabilization drives 3D centrosome migration to initiate ciliogenesis. J Cell Biol. https://doi.org/10.1083/jcb201610039.
Poole CA, Flint MH, Beaumont BW (1985) Analysis of the morphology and function of primary cilia in connective tissues: a cellular cybernetic probe? Cell Motil 5:175–193
Praetorius HA, Praetorius J, Nielsen S, Frokiaer J, Spring KR (2004) Beta1-integrins in the primary cilium of MDCK cells potentiate fibronectin-induced Ca2+ signaling. Am J Physiol Ren Physiol 287:F969–F978
Satir P. Cilia: before and after. Cilia 2017: 6, 6 (1) https://doi.org/10.1186/s13630-017-0046-8.
Schwartz EA, Leonard ML, Bizios R, Bowser SS (1997) Analysis and modeling of the primary cilium bending response to fluid shear. Am J Phys 272:F132–F138
Sherpa RT, Atkinson KF, Ferreira VP, Nauli SM (2016) Rapamycin increases length and mechanosensory function pf primary cilia in renal epithelial and vascular endothelium cells. Int Educ Res J 2:91–97
Sjöstrand FS (1953) The ultrastructure of the inner segments of the retinal rods of the guinea pig eye as revealed by electron microscopy. J Cell Comp Physiol 42:45–56
Sorokin SP (1962) Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Sci 15:363–373
Sorokin SP (1968) Reconstruction of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci 3:207–230
Stiff T, Alagoz M, Alcantara D, Outwin E, Brunner HG, Bongers EM, O'Driscoll M, Jeggo PA (2013) Deficiency in origin licensing proteins impairs cilia formation: implications for the aetiology of Meier-Gorlin syndrome. PLoS Genet 9:e1003360. https://doi.org/10.1371/journal.pgen.1003360
Tokoyasu K, Yamada E (1959) The fine structure of retina studied with the electron microscope.IV. Morphogenesis of outer segments of retinal rods. J Biophys Biochem Cytol 6:225–230
Wheatley DN (1982) The centriole: a central enigma of cell biology. Elsevier Biomedical, Amsterdam. ISBN 0-444-80359-9
Wheatley DN (1993) Oligocilia: incidence and significance in normal and pathological tissues. Ultrastructural Path 17:565–566
Wheatley DN (1995) Primary cilia in normal and pathological tissues. Pathobiology 63:222–238
Wheatley DN (2005) Landmarks in the first hundred years of primary (9+0) cilium research. Cell Biol Int 29:333–339
Wheatley DN (2008) Nanobiology of the primary cilium--paradigm of a multifunctional nanomachine complex. Methods Cell Biol 90:139–156. https://doi.org/10.1016/S0091-679X(08)00807-8
Wheatley DN, Bowser SS (2000) Measurement and length control of primary cilia: analysis of monociliates and multiciliates in PtK1 cells. Biol Cell 92:573–583
Wheatley DN, Wang A-M, Strugnell GE (1996) Expression of primary cilia in mammalian cells. Cell Biol Int 20:73–81
Yang N, Leung EL, Liu C, Li L, Eguether T, Jun Yao XJ, Jones EC, Norris DA, Liu A, Clark RA, Roop DR, Pazour GL, Shroyer KR, Chen J (2017) INTU is essential for oncogenic Hh signalling through regulating primary cilia formation in basal cell carcinoma. Oncogene 36:4997–5005
Youn YH, Han YG (2017) Primary cilia in brain development and disease. Am J Pathol. https://doi.org/10.1016/j.ajpath.2017.08.031
Zhang J, Dalbay MT, Luo X, Vrij E, Barbieri D, Moroni L, de Bruijn JD, van Blitterswijk CA, Chapple JP, Knight MM, Yuan H (2017) Topography of calcium phosphate ceramics regulates primary cilia length and TGF receptor recruitment associated with osteogenesis. Acta Biomater 57:487–497
Zimmerman KW (1898) Beiträge zur kenntniss cinigen drusen und epithelien. Arch mikrosk Anat EntwMech 52:552–576
Acknowledgements
My thanks in particular to Bernard Perbal for inviting me to contribute, albeit rather tardily, to this anniversary issue of JCCS. It would not have been possible without the help from a band of devoted researchers I have had the privilege to work with for over more than 50 years. To name them all would be difficult, as it might be iniquitous to mention only a few. However, there were two points at which my research on primary cilia was bolstered during those times when as the French say “we were talking to the wind”. One was Tony Poole, who was perhaps the strongest advocate of the primary cilium, recognizing its importance from the start of his work on it, which has been thoroughly vindicated in the interim. The other most certainly was years of working with Sam Bowser in keeping the subject alive in the 1980s. Many facets of the basic biology of primary cilia were unfolded by our combined effort. Finally I would like to thank and congratulate everyone who has jumped on board in the last two decades; their combined effort has made unexpectedly huge waves in science and medicine. I must also apologise for using only representative references to recent reports on primary cilia to make some of my points. This article is not intended as a review, but rather a personal perspective of this remarkable organelle.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wheatley, D.N. The primary cilium – once a “rudimentary” organelle that is now a ubiquitous sensory cellular structure involved in many pathological disorders. J. Cell Commun. Signal. 12, 211–216 (2018). https://doi.org/10.1007/s12079-017-0436-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-017-0436-0