Abstract
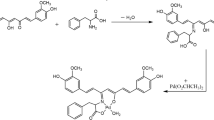
A series of coumarin-derived azolyl ethanols including imidazolyl, triazolyl, tetrazolyl, benzotriazolyl, thiol-imidazolyl and thiol-triazolyl ones were conveniently synthesized and characterized by IR, 1H NMR, 13C NMR and high-resolution mass spectra (HRMS) spectra. Some of the prepared compounds showed appropriate logPow values and effective antibacterial and antifungal activities. Noticeably, compound 14 with bis-triazolyl ethanol group exhibited low minimal inhibitory concentration (MIC) value of 8 mg/mL against MRSA, which was comparable or even superior to reference drugs Norfloxacin (MIC=8 mg/mL) and Chloramphenicol (MIC=16 mg/mL). It could also effectively inhibit the growth of the tested fungal strains compared to Fluconazole. Further binding studies of coumarin 14 with calf thymus DNA were investigated by UV-Vis absorption and fluorescence spectroscopy. It was found that compound 14 could interact with calf thymus DNA by groove binding to form 14-DNA complex via both hydrogen bonds and van der Waals force, which might be the factor to exert the powerful antimicrobial activity.
Similar content being viewed by others
References
Ferreira BS, de Almeida AM, Nascimento TC, de Castro PP, Silva VL, Diniz CG, Hyaric ML. Bioorg Med Chem Lett, 2014, 24: 4626–4629
Lv JS, Peng XM, Kishore B, Zhou CH. Bioorg Med Chem Lett, 2014, 24: 308–313
Hopkins AL, Bickerton GR, Carruthers IM, Boyer SK, Rubin H, Overington JP. Curr Top Med Chem, 2011, 11: 1292–1300
Peng XM, Cai GX, Zhou CH. Curr Top Med Chem, 2013, 13: 1963–2010
Zhang YY, Zhou CH. Bioorg Med Chem Lett, 2011, 21: 4349–4352
Alwan WS, Karpoormath R, Palkar MB, Patel HM, Rane RA, Shaikh MS, Kajee A, Mlisana KP. Eur J Med Chem, 2015, 95: 514–525
Brown GD, Denning DW, Levitz SM. Science, 2012, 336: 647
Wei JJ, Jin L, Wan K, Zhou CH. Bull Korean Chem Soc, 2011, 32: 229–238
Benincasa M, Pacor S, Wu W, Prato M, Bianco A, Gennaro R. ACS Nano, 2011, 5: 199–208
Zou Y, Yu SC, Li RW, Zhao QJ, Li X, Wu MC, Huang T, Chai XX, Hu HG, Wu QY. Eur J Med Chem, 2014, 74: 366–374
Dione N, Khelaifia S, Lagier JC, Raoult D. Int J Antimicrob Agents, 2015, 45: 537–540
Zhang L, Peng XM, Damu GLV, Geng RX, Zhou CH. Med Res Rev, 2014, 34: 340–437
Fang B, Zhou CH, Rao XC. Eur J Med Chem, 2010, 45: 4388–4398
Li LJ, Ding H, Wang BG, Yu SC, Zou Y, Chai XY, Wu QY. Bioorg Med Chem Lett, 2014, 24: 192–194
Babazadeh-Qazijahani M, Badali H, Irannejad H, Afsarian MH, Emami S. Eur J Med Chem, 2014, 76: 264–273
Wang Y, Damu GLV, Lv JS, Geng RX, Yang DC, Zhou CH. Bioorg Med Chem Lett, 2012, 22: 5363–5366
Zhang L, Kannekanti VK, Syed R, Geng RX, Zhou CH. Chem Biol Drug Des, 2015, 96: 648–655
Cui SF, Ren Y, Zhang SL, Peng XM, Damu GLV, Geng RX, Zhou CH. Bioorg Med Chem Lett, 2013, 23: 3267–3272
Kumar GVS, Rajendraprasad Y, Mallikarjuna BP, Chandrashekar SM, Kistayya C. Eur J Med Chem, 2010, 45: 2063–2074
Bonanomi G, Braggio S, Capelli AM, Checchia A, Fabio RD, Marchioro C, Tarsi L, Tedesco G, Terreni S, Worby A, Heibreder C, Micheli F. ChemMedChem, 2010, 5: 705–715
Bhatia MS, Zarekar BE, Choudhari PB, Ingale KB, Bhatia NM. Med Chem Res, 2011, 20: 116–120
Kalhor M, Mobinikhaledi A, Dadras A, Tohidpour M. J Heterocycl Chem, 2011, 48: 1366–1370
Tamilselvi A, Mugesh G. Bioorg Med Chem Lett, 2010, 20: 3692–3697
Wang QP, Zhang JQ, Damu GLV, Wan K, Zhang HZ, Zhou CH. Sci China Chem, 2012, 55: 2134–2153
Parvez A, Meshram J, Tiwari V, Sheik J, Dongre R, Youssoufi MH, Ben Hadda T. Eur J Med Chem, 2010, 45: 4370–4378
Arshad A, Osman H, Bagley MC, Lam CK, Mohamad S, Zahariluddin ASM. Eur J Med Chem, 2011, 46: 3788–3794
Patel D, Patel R, Kumari P, Patel N. Med Chem Res, 2012, 21: 1611–1624
Damu GLV, Cui SF, Peng XM, Wen QM, Cai GX, Zhou CH. Bioorg Med Chem Lett, 2014, 24: 3605–3608
Peng XM, Damu GLV, Zhou CH. Curr Pharm Des, 2013, 19: 3884–3930
Shi Y, Zhou CH. Bioorg Med Chem Lett, 2011, 21: 956–960
Shi Y, Zhou CH, Zhou XD, Geng RX, Ji QG. Acta Pharm Sinica, 2011, 46: 798–810
Zhang SL, Damu GLV, Zhang L, Geng RX, Zhou CH. Eur J Med Chem, 2012, 55: 164–175
Wang XL, Wan K, Zhou CH. Eur J Med Chem, 2010, 45: 4631–4639
Damu GLV, Wang QP, Zhang HZ, Zhang YY, Lv JS, Zhou CH. Sci China Chem, 2013, 56: 952–969
Zhang HZ, Damu GLV, Cai GX, Zhou CH. Eur J Med Chem, 2013, 64: 329–344
Zhang HZ, Wei JJ, Kannekanti VK, Rasheed S, Zhou CH. Med Chem Res, 2015, 24: 182–196
Cui SF, Peng LP, Zhang HZ, Rasheed S, Kannekanti VK, Zhou CH. Eur J Med Chem, 2014, 86: 318–334
Zhou CH, Wang Y. Curr Med Chem,, 2012, 19: 239–280
Fosso MY, Zhu HK, Green KD, Garneau-Tsodikova S, Fredrick K. ChemBioChem, 2015, doi: 10.1002/cbic.201500256
Li XL, Hu YJ. Biomacromolecules, 2012, 13: 873–880
Luo YL, Baathulaa K, Kannekanti VK, Zhou CH, Cai GX. Sci China Chem, 2015, 58: 483–494
Peng LP, Nagarajan S, Rasheed S, Zhou CH. Med Chem Commun, 2015, 6: 222–229
Yin BT, Yan CY, Peng XM, Zhang SL, Rasheed S, Geng RX, Zhou CH. Eur J Med Chem, 2014, 71: 148–159
Hu YJ, Liu Y, Xiao XH. Biomacromolecules, 2009, 10: 517–521
Sinhamahapatra A, Sutradhar A, Pahari A, Bajaj HC, Panda AB. Appl Catal A, 2011, 394: 93–100
National Committee for Clinical Laboratory Standards Approved standard Document. M27-A2, Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, National Committee for Clinical Laboratory Standards, Wayne, PA, 2002
Zhang GW, Fu P, Wang L, Hu MM. J Agric Food Chem, 2011, 59: 8944–8952
Devi CV, Singh NR. Spectrochim Acta A, 2011, 78: 1180–1186
Dai LL, Zhang HZ, Nagarajan S, Rasheed S, Zhou CH. Med Chem Commun, 2015, 6: 147–154
Zhang HZ, Lin JM, Rasheed S, Zhou CH. Sci China Chem, 2014, 57: 807–822
Lakowicz JR. Principles of Fluorescence Spectroscopy. 3rd Ed. New York: Springer, 2006. 11–12
Yang MM, Xi XL, Yang P. Chin J Chem, 2006, 24: 642–648
Zhao N, Wang XM, Pan HZ, Hu YM, Ding LS. Spectrochim Acta A, 2010, 75: 1435–1442
Ortiz M, Fragoso A, Ortiz PJ, O’Sullivan CK. J Photochem Photobiol A, 2011, 218: 26–32
Yuan JL, Liu H, Kang X, Lv Z, Zou GL. J Mol Struct, 2008, 891: 333–339
Akbay N, Seferoglu Z, Gök E. J Fluoresc, 2009, 19: 1045–1051
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Peng, XM., Kumar, K.V., Damu, G.L. et al. Coumarin-derived azolyl ethanols: synthesis, antimicrobial evaluation and preliminary action mechanism. Sci. China Chem. 59, 878–894 (2016). https://doi.org/10.1007/s11426-015-0351-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-015-0351-0