Abstract
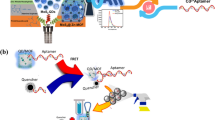
A simple and sensitive method for detection of captopril was established based on its obstructive effect on nanomaterial surface energy transfer (NSET). It was found that the acridine orange (AO) could be adsorbed onto the surface of citrated-gold nanoparticles (AuNPs) through electrostatic interaction. Incidentally, the fluorescence of AO was quenched owing to the dipole-dipole interaction of NSET between AO fluorophore and the AuNPs. However, captopril could obstruct the occurrence of NSET between AO and AuNPs effectively with the formation of Au-S covalent bonds between it and the AuNPs. Consequently, AO molecules were moved away from the surface of AuNPs leading to a decline of the energy transfer efficiency. Moreover, the fluorescence of AO could be gradually restored with the addition of captopril. Under the optimal conditions, the recovered fluorescence intensity correlated linearly with the concentration of captopril in the range of 400 nmol/L-2.0 μmol/L with a detection limit of 71 nmol/L. Besides, the proposed method was successfully applied for the detection of captopril in troches with the recovery of 93%–102% and the RSD lower than 2.24%. The results were in good agreement with those obtained from the HPLC method.
Similar content being viewed by others
References
Li M, Wang QY, Shi XD, Hornak LA, Wu NQ. Detection of mercury (II) by quantum dot/DNA/gold nanoparticle ensemble based nanosensor via nanometal surface energy transfer. Anal Chem, 2011, 83: 7061–7065
Gao F, Ye QQ, Cui P, Chen XX, Li MG, Wang L. Selective “turn-on” fluorescent sensing for biothiols based on fluorescence resonance energy transfer between acridine orange and gold nanoparticles. Anal Methods, 2011, 3: 1180–1185
Darbha GK, Ray A, Ray PC. Gold nanoparticle-based miniaturized nanomaterial surface energy transfer probe for rapid and ultrasensitive detection of mercury in soil, water, and fish. ACS Nano, 2007, 1: 208–214
Griffin J, Singh AK, Senapati D, Rhodes P, Mitchell K, Robinson B, Yu E, Ray PC. Size- and distance-dependent nanoparticle surfaceenergy transfer (NSET) method for selective sensing of hepatitis C virus RNA. Chem Eur J, 2009, 15: 342–351
Kikkeri R, Padler-Karavani V, Diaz S, Verhagen A, Yu H, Cao HZ, Langereis MA, De Groot RJ, Chen X, Varki A. Quantum dot nanometal surface energy transfer based biosensing of sialic acid compositions and linkages in biological samples. Anal Chem, 2013, 85: 3864–3870
Griffin J, Ray PC. Gold nanoparticle based NSET for monitoring Mg2+ dependent RNA folding. J Phys Chem B, 2008, 112: 11198–11201
Gholivand MB, Khodadadian M, Omidi M. Amperometric sensor based on a graphene/copper hexacyanoferrate nano-composite for highly sensitive electrocatalytic determination of captopril. Mater Sci Eng C, 2013, 33: 774–781
Hillaert S, Van den Bossche W. Determination of captopril and its degradation products by capillary electrophoresis. J Pharm Biomed Anal, 1999, 21: 65–73
Ensafi AA, Karimi-Maleh H, Ghiaci M, Arshadi M. Characterization of Mn-nanoparticles decorated organo-functionalized SiO2-Al2O3 mixed-oxide as a novel electrochemical sensor: application for the voltammetric determination of captopril. J Mater Chem, 2011, 21: 15022–15030
Siangproh W, Ngamukot P, Chailapakul O. Electrochemical determination of captopril at boron-doped diamond thin film electrode applied to a flow injection system. Sens Actuators B, 2003, 91: 60–66
Rezaei B, Damiri S. Voltammetric behavior of multi-walled carbon nanotubes modified electrode-hexacyanoferrate(II) electrocatalyst systemas a sensor for determination of captopril. Sens Actuators B, 2008, 134: 324–331
Shahrokhian S, Karimi M, Khajehsharifi H. Carbon-paste electrode modified with cobalt-5-nitrolsalophen as a sensitive voltammetric sensor for detection of captopril. Sens Actuators B, 2005, 109: 278–284
Palomeque ME, Fernadez Band BS. Flow injection biamperometric determination of captopril. J Pharm Biomed Anal, 2002, 30: 547–552
Economou A, Themelis DG, Theodoridis G, Tzanavaras PD. Sensitive determination of captopril by flow injection analysis with chemiluminescence detection based on the enhancement of the luminol reaction. Anal Chim Acta, 2002, 463: 249–255
Huang TM, He Z, Yang B, Shao LP, Zheng XW, Duan GL. Simultaneous determination of captopril and hydrochlorothiazide in human plasma by reverse-phase HPLC from linear gradient elution. J Pharm Biomed Anal, 2006, 41: 644–648
Sun YH, Zhang ZJ, Zhang XF. Determination of captopril by high-performance liquid chromatography with direct electrogenerated chemiluminescence. Spectrochim Acta A, 2013, 105: 171–175
Chen QH, Bai SL, Lu C. The new approach for captopril detection employing triangular gold nanoparticles-catalyzed luminol chemiluminescence. Talanta, 2012, 89: 142–148
Rodrigues SSM, Santos JLM. Chemiluminometric determination of captopril in a multi-pumping flow system. Talanta, 2012, 96: 210–215
Murillo Pulgarin JA, Garcia Bermejo LF, Lopez PF. Sensitive determination of captopril by time-resolved chemiluminescence using the stopped-flow analysis based on potassium permanganate oxidation. Anal Chim Acta, 2005, 546: 60–67
Gouda AA, Amin AS. Copper (II)-neocuproine reagent for spectrophotometric determination of captopril in pure form and pharmaceutical formulations. Arabian J Chem, 2010, 159–165
El-Didamony AM, Eman Erfan AH. Utilization of oxidation reactions for the spectrophotometric determination of captopril using brominating agents. Spectrochim Acta A, 2010, 75: 1138–1145
Wang SL, Wang M, Li QM. Application of potassium ferricyanide in the spectrophotometric determination of captopril. Chin Chem Lett, 2009, 20: 88–91
Gao LX, Wu LL, Li QM. Catalysis reaction between sodium 1, 2-naphthoquinone-4-sulfonate and hydroxyl ion using captopril as catalyzer and determination of captopril. Anal Chim Acta, 2008, 626: 174–179
Liu ZD, Huang CZ, Li YF, Long YF. Enhanced plasmon resonance light scattering signals of colloidal gold resulted from its interactionswith organic small molecules using captopril as an example. Anal Chim Acta, 2006, 577: 244–249
Hormozi-Nezhad MR, Bagheri H, Bohloul A, Taheri N, Robatjazi H. Highly sensitive turn-on fluorescent detection of captopril based on energy transfer between fluorescein isothiocyanate and gold nanoparticles. J Lumin, 2013, 134: 874–879
Grabar KC, Freeman RG, Hommer MB, Natan MJ. Preparation and characterization of collid monolayers. Anal Chem, 1995, 67: 735–743
Jin RC, Wu GS, Li Z, Mirkin CA, Schatz GC. What controls the melting properties of DNA-linked gold nanoparticle assemblies? J Am Chem Soc, 2003, 125: 1643–1654
Gao F, Ye QQ, Cui P, Chen XX, Li MG, Wang L. Selective “turn-on” fluorescent sensing for biothiols based on fluorescence resonance energy transfer between acridine orange and gold nanoparticles. Anal Methods, 2011, 3: 1180–1185
Chen YM, Cheng TL, Tseng WL. Fluorescence turn-on detection of iodide, iodate and total iodine using fluorescein-5-isothiocyanatemodified gold nanoparticles. Analyst, 2009, 134: 2106–2112
Aykin N, Neal R, Yusof M, Ercal N. Determination of captopril in biological samples by high performance liquid chromatography with ThioGlo 3 derivatization. Biomed Chromatogr, 2001, 15: 427–432
Chen SJ, Chang HT. Nile red-adsorbed gold nanoparticles for selective determination of Thiols based on energy transfer and aggregation. Anal Chem, 2004, 76: 3727–3734
Li M, Cushing SK, Wang QY, Shi XD, Hornak LA, Hong ZL, Wu NQ. Size-dependent energy transfer between CdSe/ZnS quantum dots and gold nanoparticles. J Phys Chem Lett, 2011, 2: 2125–2129
Sadhu S, Patra A. Donor-acceptor systems: energy transfer from CdS quantum dots/rods to Nile red dye. ChemPhysChem, 2008, 9: 2052–2058
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, J., Yang, Y., Hu, X. et al. Spectrofluorimetric analysis of captopril based on its obstruction effect of the nanomaterial surface energy transfer between acridine orange and gold nanoparticles. Sci. China Chem. 58, 885–891 (2015). https://doi.org/10.1007/s11426-014-5291-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-014-5291-8